Lysozyme-catalyzed formation of a conjugated polyacetylene†
Abstract
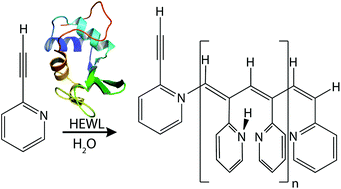
Hen egg white lysozyme catalyzes the polymerization of 2-ethynylpyridine in water as the singular protein catalyst. Inhibition and NMR spectroscopy studies support the hypothesis that polymerization takes place in the active site cleft of the protein. This discovery marks the first time a metal-free hydrolase has been observed activating the formation for a conjugated polymer.


 Please wait while we load your content...
Please wait while we load your content...