The origin of bisignate circularly polarized luminescence (CPL) spectra from chiral polymer aggregates and molecular camphor: anti-Kasha's rule revealed by CPL excitation (CPLE) spectra†
Abstract
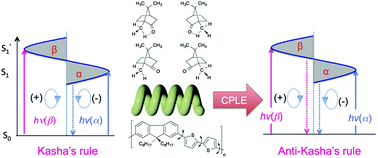
The first circularly polarized luminescence (CPL) excitation (CPLE) measurements of poly(fluorene-alt-bithiophene) (PF8T2) aggregates with bisignate CPL/circular dichroism (CD) signals and, for comparison, bisignate CPL signal camphor were achieved. Based on this finding, we propose that Kasha's rule can be broken in chiral luminophore systems. The |gem| values of PF8T2 hetero-aggregate with help of helical polysilanes (PSi-S/-R) as sacrificial scaffoldings boosted to 0.05–0.08 at ≈510 and ≈540 nm associated with a high quantum yield of 0.33. The gem values arose from hugely amplified |gabs| values of 0.15–0.25 at ≈500 nm and ≈510 nm. Moreover, PF8T2 homo-aggregate, maintaining the bisignate CPL and CD spectral profiles, was generated by PSi-S/-R selective photoscissoring reaction at 313 nm for 10 s.


 Please wait while we load your content...
Please wait while we load your content...