A comprehensive kinetic study of the conventional free-radical polymerization of seven-membered cyclic ketene acetals†
Abstract
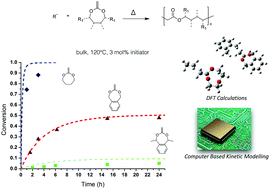
The current study reports on the kinetic analysis of the free-radical polymerization of several seven-membered cyclic ketene acetal monomers and in particular 2-methylene-1,3-dioxepane (MDO) and 5,6-benzo-2-methylene-1,3-dioxepane (BMDO). Such monomers are known to undergo a complete ring-opening to afford polyester chains by a radical pathway. The influence of experimental conditions (e.g., nature and concentration of the initiator, temperature, solvent, etc.) on the kinetics and molar mass distribution was studied. Whereas it was shown that BMDO cannot be polymerized using peroxides due to H-abstraction, the experimental results also demonstrated that the polymerization kinetics were strongly driven by the nature of the propagating radical, with reactivity order: MDO > BMDO > 4,7-dimethyl-5,6-benzo-2(chloromethyl)-1,3-dioxepane (MeBMDO). The copolymerization of MDO and BMDO (1 : 1) was also investigated. The incorporation of both monomers was identical but the polymerization kinetics were governed by the monomer with the lowest reactivity. The experimental results were finally rationalized by the combination of DFT calculations of the propagation rate constants with PREDICI modelings.
- This article is part of the themed collection: Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...