Ag(Ag2O)–ZrO2–Y2O3 photosensitive composites: influence of synthesized routes on structure and properties
O.
Gorban
*,
I.
Danilenko
,
S.
Gorban
,
G.
Volkova
,
V.
Glazunova
and
T.
Konstantinova
Materials science department, Donetsk institute for physics and engineering NAS of Ukraine, Kyiv, Nauki av. 46, 03680 Ukraine. E-mail: oxanag1@ukr.net
First published on 11th November 2016
Abstract
It was shown that Ag(or Ag2O)–ZrO2–Y2O3 formation occurs due to the complex process of decomposition and structure transformation of oxide materials and Ag-complexes. Differences in the decomposition temperatures of Ag-complexes, in particular, and their melting temperatures, and the transformation of ZrO2–Y2O3 NPs morphology leads to the creation of composite structure of two types: an Ag–NPs/zirconia matrix and zirconia core/Ag-shell. It was shown that for these composites the temperature is an effective approach for controlling the NPs sizes for both components (Ag clusters and zirconia NPs), the defectiveness of the complex oxide and their optical properties, in particular the photosensitive to visible irradiation range.
1. Introduction
Heterogeneous systems designers have a tendency to use complex composite structures where special properties of the interfaces are important.1 The creation of metal/oxide composite materials may significantly enhance basic oxide materials’ functionality, in particular the optical, electrochemical, conductivity and photosensitivity,2 due to the possibility of reversible electron transfer between components of the composite at excitation of those materials in different irradiation ranges. Ag or silver dioxide (Ag2O)-modified zirconia are promising composite structures for chemistry, environmental media, medicine, the electronics industry and other fields.3Silver (Ag), gold (Au) and platinum (Pt) are often used as metal components for surface decoration of wide band gap semiconductor materials, such as titanium dioxide (TiO2), zinc oxide (ZnO) and zirconium dioxide (ZrO2). In practice, composite materials containing Ag NPs and oxide (NPs or matrix) are created by a variety of methods; for example low-energy ion beam deposition, sol–gel, direct metal implanting, light ion irradiation of an ion exchange oxide matrix, evaporation–condensation, using a very exotic leaf extract assisted bioreduction process, and other methods.4–7
Usually, mono- or polycrystalline oxide particles and films are used as substrates (or matrix) for the implantation of metals to the oxides for the creation of composite materials.4,5,7,8 The usage of energy in the technology of creating metal/oxide can lead to significant changing in the surface state and lattice of the modified oxide. Thus, the authors9 showed that irradiation of cubic zirconia monocrystal by noble metal ions led to incorporation of ions into crystals with a penetration thickness up to 1.5 μm and to form a complex of noble metal with Zr ions of the crystal lattice. These complexes were destroyed with subsequent heat-treatment of the composite material.
For zirconia, which has some polymorphs (monoclinic, tetragonal or cubic), the development of technologies to create noble metal/oxide without high-energy actions on the oxide component is a difficult task. Wet chemistry methods are cheaper variants for the synthesis of noble/metal composite and allow the controlling of structures and properties of the composite by changing the electronic state of Ag in the oxide matrix or oxide polymorph by varying the synthesis conditions, in particular, temperature or reduction type.7,8,10–17 Thus, the oxygen vacancies in a tetragonal zirconia crystal leads to the formation of a surface state in which Ag ions are included in the complex with neighbouring oxygen vacancies and the dispersion of Ag is higher in this phase than it is in the monoclinic phase.11 The changing of zirconia matrix to alumina, to obtain Ag metal nanoparticles (NPs) in an oxide is practically impossible because the Ag ions build in the crystal lattice and form aluminium–oxygen–silver (Al–O–Ag) bonds up to 900 °C.17 In contrast, when creating a silver–germanium dioxide (Ag–GeO2) composite structure, the temperature is a factor for controlling the size of the Ag NPs, and therefore the use of a low temperature range (150 °C–350 °C) leads to the formation of Ag NPs with a wide size distribution (the size of the Ag NPs changes from 5 nm to 50 nm as the temperature changes from 150 °C to 350 °C).18 The authors7 also showed that the calcination of sol–gel films Ag/ZrO2 in the low temperature range of 200 °C–300 °C leads to the formation of two set sizes of Ag0 NPs in the amorphous zirconia films, which are 20 and 80 nm; plasmonic resonance in these systems was observed at wavelengths about 450 and 500 nm, respectively. Investigation of the influence of the amount of Ag on the structure and optical properties of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZrO2 thin films, which were formed at 300 °C, showed the dependence of Ag NPs size on the silver quantity. It was shown that the increasing of Ag loading in zirconia matrix led to the growth of the Ag NPs size from 7 to 20 nm and estimated wavelengths for two extreme values were 470 nm and 520 nm.8 The optical spectra of Ag NPs in sol–gel films that were formed at a higher temperature range of 500 °C–600 °C showed the presence of two peaks at 470 and 520 nm with an intensity dependence on the temperature used.12 The plasma frequency, which was estimated for zirconia materials, corresponds to 452 nm.4 Such optical properties of Ag/zirconia strongly depended on the synthesis history and evolution of Ag NPs with heat-treatment, which is described as a set of sequential processes, such as the interaction of Ag NPs with an oxide matrix, the destruction of a complex oxide and the formation of Ag NPs with a narrower size distribution on the oxide surface.14 X-ray photoelectron spectroscopy (XPS) investigation showed that, for Ag-modified zirconia, temperature may be used to create an Ag shell/zirconia core type composite.12 At the same time, under photoreduction conditions, different electronic states of Ag in the composite can be observed. The XPS shows the presence of silver(I) oxide (Ag2O) (Ag 3d5/2 = 367.7 eV), Ag (368.4 eV) and a transition charge state (368.8 eV),12,13 and for another case, electron spin resonance (ESR)15,16 has been shown to exist in the creation of Ag+–Ag0 clusters in bulk or on the surface of the oxide particles. In other words, the type of oxide matrix, the temperature of the treatment and the reduction conditions may be important factors for controlling the composite morphology, charge state and dispersity of Ag NPs and their properties.
ZrO2 thin films, which were formed at 300 °C, showed the dependence of Ag NPs size on the silver quantity. It was shown that the increasing of Ag loading in zirconia matrix led to the growth of the Ag NPs size from 7 to 20 nm and estimated wavelengths for two extreme values were 470 nm and 520 nm.8 The optical spectra of Ag NPs in sol–gel films that were formed at a higher temperature range of 500 °C–600 °C showed the presence of two peaks at 470 and 520 nm with an intensity dependence on the temperature used.12 The plasma frequency, which was estimated for zirconia materials, corresponds to 452 nm.4 Such optical properties of Ag/zirconia strongly depended on the synthesis history and evolution of Ag NPs with heat-treatment, which is described as a set of sequential processes, such as the interaction of Ag NPs with an oxide matrix, the destruction of a complex oxide and the formation of Ag NPs with a narrower size distribution on the oxide surface.14 X-ray photoelectron spectroscopy (XPS) investigation showed that, for Ag-modified zirconia, temperature may be used to create an Ag shell/zirconia core type composite.12 At the same time, under photoreduction conditions, different electronic states of Ag in the composite can be observed. The XPS shows the presence of silver(I) oxide (Ag2O) (Ag 3d5/2 = 367.7 eV), Ag (368.4 eV) and a transition charge state (368.8 eV),12,13 and for another case, electron spin resonance (ESR)15,16 has been shown to exist in the creation of Ag+–Ag0 clusters in bulk or on the surface of the oxide particles. In other words, the type of oxide matrix, the temperature of the treatment and the reduction conditions may be important factors for controlling the composite morphology, charge state and dispersity of Ag NPs and their properties.
At the same time, temperature is also a factor for controlling the size, morphology, surface state and fractal dimension of the NPs in a zirconia matrix, as well as the degree of connection that the NPs have with each other.19,20 Zirconia is formed from amorphous zirconium hydroxide as a result of the sequential processes of dehydration (up to 300 °C), crystallization (400 °C–500 °C), destruction of the amorphous matrix (400 °C–500 °C) and dispersion of the zirconia NPs (above the crystallization temperature). It is also known that different zirconia salts have different melting and decomposition temperatures, so argentum nitrate has a lower melting temperature (209 °C) and decomposition (near 350 °C) than argentum chloride (melting near 450 °C). We can see that the Ag complexes that are built using different ligands (nitrate (NO3−) and chlorine (Cl−) ions) will also have different melting and decomposition temperatures. This provides the possibility to create a composite structure based on zirconia with Ag2O or metallic Ag NPs, which are built upon new synthesis principles. In this approach, silver complexes with different structures are used as precursors. Silver complex ligands can consist of ammonium groups, hydroxyl groups, water or other inorganic ions. One of the features of the realised synthesis is the simultaneous formation of complex oxide and silver complexes as a common system in a co-precipitation process, and in its subsequent thermal treatment. This study presents different routes that can be used to prepare a composite structure based on zirconia that allow variations not only in the morphology of the system and the size of the particles or clusters of the separate components of the composites, but also their photosensitive activity. Thus, when creating these types of composite structures questions arise about the structure of the oxide component, the electronic state of the metal, the changes based on variations in temperature or the reduction treatments and the interrelationship between their characteristics and the photosensitive properties of the composites.
2. Experimental
ZrO2–Y2O3–Ag2O composites were synthesized with a co-precipitation technique using ZrOCl2·nH2O or (ZrO(NO3)2), Y(NO3)3 and AgNO3 salts. The amount of zirconium salt was 0.87 M. The amount of Y(NO3)3 was used in a quantity that was equal to 3 mol% as calculated for Y2O3 compared to ZrO2. The amount of AgNO3 was used in a quantity that was equal to 1 mol% as calculated for Ag2O compared to ZrO2. All used chemicals were of chemical grade purity. The water solutions of salts were mixed together on a propeller and stirred for 30 min. The precipitated gel was obtained by adding an aqueous solution of ZrOCl2·nH2O or (ZrO(NO3)2), Y(NO3)3 and AgNO3 in NH4OH aqueous water solution with continuous stirring. The pH value was 8. The gelation was continued for one hour at room temperature. After that the precipitate was recovered by filtration with a vacuum pump. The gel was washed by distilled water at pH 6. After washing and filtration, the hydrogel was dried in a microwave furnace with a 700 W output power and at a 2.45 GHz frequency. The dried hydroxides were calcined in a resistive furnace at different temperatures with a 2 h dwelling time. For the reduction of Ag in the Ag2O–ZrO2–Y2O3 composite structure, the following procedures were used:- a suspension was of Ag2O–ZrO2–Y2O3 powders was prepared in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio at pH 8;
3 ratio at pH 8;
- a reducing agent solution of 0.04 M glucose was prepared;
- the suspension and glucose solution were mixed with each other;
- the Ag NPs were reduced with 700 W under MW irradiation for 10 minutes, and then the suspension was filtered;
- the Ag–ZrO2–Y2O3 composite was dried in a furnace at 140 °C.
The powders were characterized by XRD (Dron-3) with Cu-Kα radiation for crystallite sizes and quantitative phase analyses. Particle sizes of different calcined powders were estimated by means of transmission electron microscopy (JEM 200, Jeol, Japan). The photosensitive properties of Ag2O–ZrO2–Y2O3– nanopowders were measured on a Cary 5000 UV-Vis-NIR spectrometer (Agilent Technologies, USA). The ESR investigation was carried out with an ESR spectrometer CMS-8400 (Adani, Belarus).
3. Results and discussion
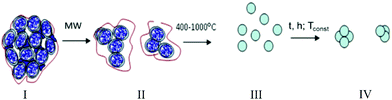
The choice of the oxide matrix (ZrO2–3 mol% Y2O3) was determined by the possibility of creating a tetragonal phase with oxygen vacancies that allows the formation of smaller silver clusters on the surface or in the matrix of the tetragonal zirconia in comparison to other polymorphic forms.11 As noted above, the type of synthesis method used enables one to obtain the oxide materials as amorphous or crystalline nanoparticles (NPs) with different sizes and surface states, as a result of the set of processes that are applied. The scheme used for the formation of the oxide matrix at different heat-treatments is presented in Fig. 1.In the process of drying under microwave (MW) irradiation, the amorphous zirconium hydroxide hydrogel (Zr(Y)O(OH)2·nH2O) lost water that was condensed in the porous hydrogel (up to 70%). According to the differential scanning calorimetry (DSC) data, the heating of amorphous zirconia (ZrO2–Y2O3·(n − x)H2O) allowed the future loss of the physically- and chemically-adsorbed water; the water was removed more quickly when the temperature range was 50 °C–300 °C (up to 29 wt%), and water was removed more slowly when the temperature was increased to 700 °C (the water quantity was 46 wt%). In the temperature range of 400 °C–500 °C, the amorphous zirconia crystallites (ZrO2–Y2O3) were formed. The transfer to isothermal regimes led to an increase in the size of the oxide matrix NPs with increasing calcination temperature.19 As can be seen, amorphous zirconium hydroxide was formed by the amorphous zirconia NPs connected to each other, and the structure could be described as porous. The crystalline zirconia NPs were formed at temperatures above the crystallization temperature, and the system could be described as dispersive. Thus, the zirconia matrix had a different morphology, and it showed the transfer from a porous structure to a dispersive system with different NPs sizes and degree of connection depending on the calcination temperature.20,21
A. Features of the formation of the composite structure in amorphous zirconia matrix
One features of the determined Ag2O–ZrO2–Y2O3 synthesis is the simultaneous formation of complex oxide and silver complexes as a common system in the co-precipitation process and its subsequent thermal treatment.In this approach, silver complexes were used as precursors in contrast to the traditional scheme of preparing catalysts. Ligands can be ammonium groups, hydroxyl groups, water or other inorganic ions. Differences in the silver complex structure led to variation in the decomposition temperature. Two processes, the zirconia matrix formation and silver complex decomposition or melting occurred simultaneously. This allowed for variations not only in the morphology of the system, but also in the sizes of the particles or clusters of the separate components of the composites.
Fourier transform infrared spectroscopy (FTIR) of the synthesized amorphous systems showed a set of absorption bands (3167 cm−1 and 3050 cm−1) and narrow bands with frequencies at 763 cm−1 and 826 cm−1 (Fig. 2), demonstrating the formation of ammonium complexes of silver [Ag(NH3)2−m(H2O)m]X, where X is either the chloride (Cl−) or nitrate (NO3−) ion depending on the type of zirconium salt that is used in the synthesis (Fig. 2). The presence of bands at 487 cm−1 and 647 cm−1 is assigned to the zirconium–oxygen–zirconium (Zr–O–Zr) bonds that are oriented in the tetragonal phase. In the ESR spectra, the signal from Ag0 is absent for such systems.
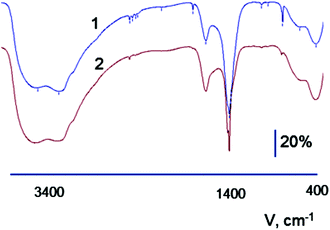 | ||
| Fig. 2 FTIR spectra of Ag2O–ZrO2–Y2O3 amorphous composite which was synthesized based on salts (1) nitrate, (2) chloride. | ||
It is known that the structure of amorphous zirconia is porous regardless of the type of zirconium salt that is used in the synthesis16,17 because the reduction of silver may be occurring in the pores. The ESR data for Ag–ZrO2–Y2O3 confirm our proposition (Fig. 3).
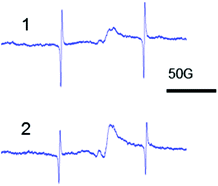 | ||
| Fig. 3 ESR spectra of amorphous Ag–ZrO2–Y2O3 which synthesized from zirconium salt: (1) nitrate, (2) chloride. | ||
The isotropic signal with giso = 2.003 in the ESR spectra corresponds to the formation of Ag0 NPs.22 The ESR data provides evidence of the formation of Ag/ZrO2–Y2O3 composites, which has the structure of Ag NPs in the porous ZrO2–Y2O3 matrix (Fig. 3). It should be noted that the isotropic signal corresponding to Ag0 NPs in the ESR spectra of the non-reduced Ag2O–ZrO2–Y2O3 amorphous systems was not observed.
B. Features of the formation of the composite structure in crystalline zirconia
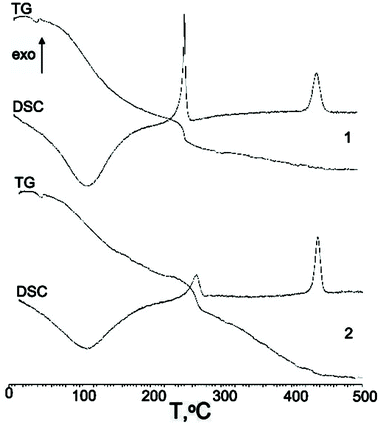 | ||
| Fig. 4 DSC data of Ag2O–ZrO2–Y2O3 systems that are synthesized based on (1) chloride (2) nitrate salts. | ||
Based on the DSC data, three processes, one endothermic and two exothermic, can be observed during the heating of the prepared amorphous systems. The endothermic process corresponds to dehydration, and it occurred at temperatures up to 200 °C. The maximum temperatures for these processes were practically equal (near 115 °C) for both systems, and the difference in the enthalpies of dehydration of these processes was near 1.8 kJ mol−1. It is important to note that the dehydration parameters for the ZrO2–Y2O3 and Ag2O–ZrO2–Y2O3 systems that were synthesised with nitrate and chloride zirconium salts were likely to change.
The first exothermal peak was observed at 253 °C and 263 °C for the systems that were synthesised from zirconium chloride and nitrate, respectively. The process may correspond to the destruction or rebuilding of [Ag(NH3)2−m(H2O)m]X (X = Cl− or NO3−), in particular the removal of water, ammonium and the nitrate groups, and the formation of argentum oxide. Chloride ions were removed at higher temperatures (up to 900 °C). The enthalpies of these processes differed (105 J g−1 when using chloride salts in contrast to 55 J g−1 when using nitrate salts), and it may be connected to the difference in the stability of the Ag complex that occurs when using chloride and nitrate ligands.
Thermogravimetric (TG) investigations (TG data, see Fig. 4) show that the systems lost approximately an equal amount of water when heated up to 500 °C (30% and 34% when chloride and nitrate salts at synthesis, respectively). In the first step of dehydration up to 225 °C, the amount of water loss was 20% and 15% for chloride and nitrate, respectively. This is a feature of the formation of the oxide matrix, which was synthesised from the chloride salts. In the second stage (225 °C–280 °C), the amount of lost water was 6%–7%. The residual amount of water was lost when the temperature increased up to 700 °C.
The second exothermal peak corresponds to the crystallisation of the amorphous oxide matrix. The difference in the maxima of the crystallisation temperature was very small (about 5 °C), and the enthalpies for the crystallisation were practically equal (107 J g−1 and 109 J g−1 for nitrate and chloride, respectively). This provides evidence that the ordering in the formed crystal (defectiveness) was practically equal.
The XRD data confirmed these results and showed that all the Ag2O–ZrO2–Y2O3 crystals were tetragonal. However, silver oxide traces were found in the systems that were synthesised using chloride technology.
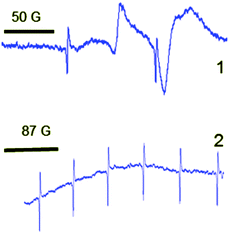 | ||
| Fig. 5 ESR spectra of crystalline composite Ag–ZrO2–Y2O3 that synthesized from zirconium salt (1) nitrate, (2) chloride. | ||
For the Ag–ZrO2–Y2O3 case, which was obtained using chloride technology, the well-separated signal with giso = 2.003 for Ag0 in the ESR spectra was not observed and the signal of Zr3+ was also absent. The ESR spectra results demonstrate a broad magnetic field signal range of 3150–3600 G. This signal may correspond to very small silver clusters, which are localised on the surface of the NPs. This may provide evidence of the formation of composite materials with a core/shell structure.
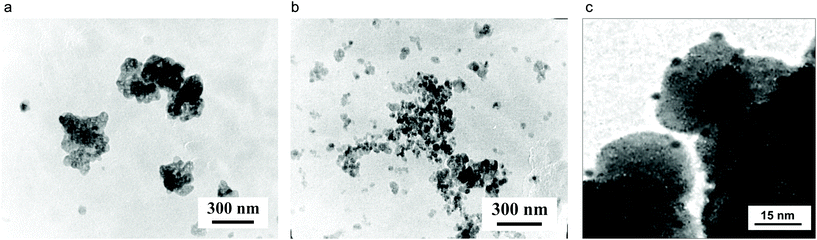
Transmission electron microscopy (TEM) investigations of the prepared reduced Ag–ZrO2–Y2O3 systems showed differences in the morphologies (Fig. 6), and confirmed that the proposed composite had an Ag0 shell/ZrO2–Y2O3 core when the composite was formed using the chloride route for the synthesis and an Ag0 NPs in ZrO2–Y2O3 porous matrix when it is formed using the nitrate synthesis route. Detailed analysis of TEM data for the Ag–ZrO2–Y2O3 systems (Tcalc = 700 °C followed by reduction) (Fig. 6c) showed two sets of Ag0 NPs present on the ZrO2–Y2O3 NPs surface, in particular, the sizes of the first type of Ag0 NPs were about 1–1.5 nm and Ag0 NPs of second type had a bigger size (about 4–5 nm). The first kind of Ag0 NPs formed a shell of ZrO2–Y2O3 core and the large Ag0 NPs had a decorated surface of composite.
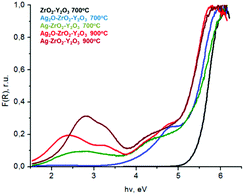 | ||
| Fig. 7 UV-visible spectra of composite systems Ag(Ag2O)–ZrO2–Y2O3 which was synthesized by chloride technology with different regime of heat treatment. | ||
Analyses of these data in the Kubelka–Munka function (F(R)) showed that for composite systems, the red shift of edge of the fundamental absorption was observed in comparison with the ZrO2–Y2O3 system, see Fig. 7. The value of this shift for Ag2O–ZrO2–Y2O3 and for Ag–ZrO2–Y2O3 depended on their calcination temperature.
Thus for the ZrO2–Y2O3 tetragonal crystal with size 18 nm (Tcalc = 700 °C) the) fundamental absorption edge value was 5.5 eV, but when transformed to the Ag2O shell/ZrO2–Y2O3 core systems, this values shifted to 5.2 and 4.7 eV for systems obtained at 700 °C and 900 °C calcination temperatures, respectively. The reduction of Ag in these systems did not change the edge of fundamental absorption values, see Fig. 7. In addition to the absorption of photons above the fundamental absorption edge of a complex oxide ZrO2–Y2O3 core (5.2 eV) in the spectrum of Ag2O–ZrO2–Y2O3 (700 °C), there is a fundamental absorption edge of Ag2O (with a 3.9 eV energy). For the Ag2O–ZrO2–Y2O3 (900 °C) system in addition to absorption bands, which were mentioned above, the plasmonic absorption of photons with energies of 2.4 eV (515 nm) and 3.2 eV (384 nm) were observed. This is connected with the decomposition of Ag2O near this temperature. Optical properties of composite NPs are due to the interaction of incident waves and electromagnetic oscillations, which are induced by dipoles. NPs may be described as dipoles because their sizes are less than the wavelength of an incident wave, and for estimation of their optical properties, the dipole quasi-static approach may be used. In this case, the quantitative characteristic is complex polarizability and for spherical NPs, polarizability may be estimated as follows:23
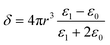 | (1) |
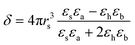 | (2) |
| εa = εc(3 − 2P) + 2εsP | (3) |
| εb = εc + εs(3 − P) | (4) |
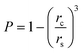 | (5) |
| σa = kIm(δ) | (6) |
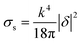 | (7) |
Spherical NPs with sizes of 1–20 nm have one peak of plasmonic resonance if the interaction between NPs does not occur.
All our systems showed two peaks plasmon resonance (Fig. 7). The first peak near 3.2 eV may correspond to the Ag0 spherical NPs with 4–5 nm size, which decorated the composite surface (Fig. 6c). Similar (6–7) NPs sized up to 15 nm have plasmon resonance at the same wavelength. The appearance of second peaks at 2.4–2.8 eV may be due to the same reasons; the first creation of core/shell structure with a thin Ag shell on a ZrO2–Y2O3 core and strong electromagnetic interaction between Ag NPs in shell were confirmed by ESR spectroscopy. The peak shift at 2.4 eV towards a lower wavelength after reduction of silver by glucose demonstrated an increasing shell density in such composites. For example, Fig. 8 shows a theoretical spectra of the cross-section scattering versus wavelength that are estimated by eqn (1)–(7) for the composite structure. The estimated average Ag NPs size was 4–5 nm and the thickness of the Ag shell was about 1.3–1.5 nm.
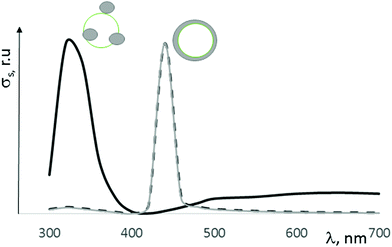 | ||
| Fig. 8 Estimated cross-scattering values for different types of structures of Ag–ZrO2–NPs with a calcination temperature of 900 °C. | ||
Reduction treatment of the Ag2O–ZrO2–Y2O3 composite structure led to the formation of Ag0 clusters on an oxide NPs surface that absorbed radiation with 2.8 eV (440 nm) and 3.2 eV (384 nm) energies in the UV-visible range. For all composite systems below the edge of fundamental adsorption, we observed a tail in the spectrum (range 4–5 eB). This possibility corresponds to both Ag2O traces in the composites and surface defects of ZrO2–Y2O3, which were created due to the solution of silver ions in zirconia.
4. Conclusion
The formation of Ag(or Ag2O)–ZrO2–Y2O3 occurs due to the complex process of decomposition and structure transformation of oxide materials and Ag-complexes. Differences in structure and stability of Ag-complexes, in particular their melting temperatures, and also dependence of oxide NPs morphology from calcination temperature allowed two types of composite structures to be realized: Ag(Ag2O)–NPs/in zirconia matrix and Ag(Ag2O)–shell zirconia core. It was shown that for these composites, temperature is an effective approach for controlling of NPs sizes of both components (Ag clusters and zirconia NPs), defectiveness of the composite and their optical properties, in particular the photosensitivity to visible irradiation range.Acknowledgements
The authors are thankful for the H2020-MSCA-RISE-2015 Programme, the project N690968 NANOGUARD2AR and project N 47/15H of program NAS of Ukraine “Fundamental problems of creation of new nanomaterials and nanotechnologies” for financial support of their work.Notes and references
- Y. Ju-Nam and J. R. Lead, Sci. Total Environ., 2008, 400, 396 CrossRef CAS PubMed.
- Nanocomposites and polymers with analytical methods, ed. J. Cuppoletti, Rijeka, In Tech, 2011, p. 51 Search PubMed.
- D. Cui, F. Tian, S. R. Coyer, J. Wang, B. Pan, F. Gao, R. He and Y. Zhang, J. Nanosci. Nanotechnol., 2007, 7, 1639 CrossRef CAS PubMed.
- Y. Saito, Y. Imamura and A. Kitahara, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 206, 272 CrossRef CAS.
- T. Fujita, K. Ijima, N. Mitsui, K. Mochiduki and Y. Saito, Jpn. J. Appl. Phys., 2007, 46, 7362 CrossRef CAS.
- S. Vivekanmandhan, M. Venkateswardlu, U. Rawls, M. Misra, A. Mohanty and N. Satyanarayaka, Ceram. Int., 2015, 41, 3305 CrossRef.
- E. Yokoyama, H. Sakata and M. Wakaki, J. Mater. Res., 2009, 24(8), 2541 CrossRef CAS.
- M. Kumar, P. K. Kulriya, J. C. Pivin and D. K. Avasthi, J. Appl. Phys., 2011, 109, 044311 CrossRef.
- O. Gorshkov, M. Schenina, A. Kasatkin, D. Pavlov, I. Antonov, A. Bobrov and D. Filatov, Letters in JTF, 2015, 41(11), 62 Search PubMed.
- Lj. Kundokovic and M. Flytrani-Stephanopoulos, Appl. Catal., A, 1999, 183, 35 CrossRef.
- R. Grabowski, J. Słoczyński, M. Śliwa, D. Mukha, R. P. Socha, M. Lachowska and J. Skrzypek, ACS Catal., 2011, 1(4), 266 CrossRef CAS.
- A. Eremenko, N. Smirnova, Iu. Gnatiuk, O. Linnik, N. Vityuk, Yu. Mukha and A. Korduban, Chapter in Book 3: Composite Materials, 2011, p. 2 Search PubMed.
- J. Matsuoka, R. Naruse, H. Nasu and K. Kamiya, J. Non-Cryst. Solids, 1997, 218, 151 CrossRef CAS.
- F. Yang, X. Jing, J. Huang, D. Sun and Q. Li, Ind. Eng. Chem. Res., 2015, 54, 20 Search PubMed.
- M. Kumar and G. B. Reddy, Plasmonics, 2016, 11(1), 261 CrossRef CAS.
- W. Gruenert, A. Brueckner, H. Hofmeister and P. Claus, J. Phys. Chem. B, 2004, 108, 5709 CrossRef CAS.
- X. She and M. Flytzani-Stephanopoulos, J. Catal., 2006, 237, 79 CrossRef CAS.
- G. E. Malashkevich, G. P. Shevchenko, S. V. Serezhkina and P. P. Pershukevich, in The 13th Int. Workshop on Sol–Gel Science and Technology, University of California, Los Angeles, 2005, p. 415.
- T. Konstantinova, I. Danilenko, V. Glazunova, G. Volkova and O. Gorban, J. Nanopart. Res., 2011, 13, 4015 CrossRef CAS.
- O. Gorban, S. Synyakina, G. Volkova, Y. Kulik and T. Konstantinova, High Pressure Res., 2011, 32(1), 72 CrossRef.
- O. A. Gorban, S. A. Sinyakina, Yu. O. Kulik, T. A. Ryumshina, S. V. Gorban, I. A. Danilenko and T. E. Konstantinova, Funct. Mater., 2010, 17(4), 1 Search PubMed.
- A. D. Stevens and M. C. R. Symons, Chem. Phys. Lett., 1984, 109, 514 CrossRef CAS.
- V. Klimov, Nanoplasmonics, CRC Press Tailor and Francis group, Brooken, 2014, p. 593 Search PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2017 |