Rhodium-catalyzed benzoisothiazole synthesis by tandem annulation reactions of sulfoximines and activated olefins†
Abstract
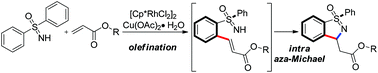
Rhodium(III)-catalyzed N-directed ortho C–H activation reactions have been developed for the synthesis of unique heterocyclic benzoisothiazoles. Herein, this novel tandem annulation approach can efficiently construct benzoisothiazole compounds from free NH-sulfoximines and alkenes via C–H activation, olefination and subsequent intramolecular aza-Michael cyclization.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...