β-Alanine and N-terminal cationic substituents affect polyamide–DNA binding†
Abstract
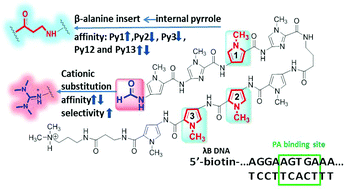
Minor-groove binding hairpin polyamides (PAs) bind specific DNA sequences. Synthetic modifications can improve PA–DNA binding affinity and include flexible modules, such as β-alanine (β) motifs to replace pyrroles (Py), and increasing compound charge using N-terminal cationic substituents. To better understand the variations in kinetics and affinities caused by these modifications on PA–DNA interactions, a comprehensive set of PAs with different numbers and positions of β and different types of N-cationic groups was systematically designed and synthesized to bind their cognate sequence, the λB motif. The λB motif is also a strong binding promoter site of the major groove targeting transcription factor PU.1. The PA binding affinities and kinetics were evaluated using a spectrum of powerful biophysical methods: thermal melting, biosensor surface plasmon resonance and circular dichroism. The results show that β inserts affect PA–DNA interactions in a number and position dependent manner. Specifically, a β replacement between two imidazole heterocycles (ImβIm) generally strengthens binding. In addition, N-terminal cationic groups can accelerate the association between PA and DNA, but the bulky size of TMG can cause steric hindrance and unfavourable repulsive electrostatic interactions in some PAs. The future design of stronger binding PA requires careful combination of βs and cationic substituents.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...