Synthesis of carbazole-based BODIPY dimers showing red fluorescence in the solid state†
Abstract
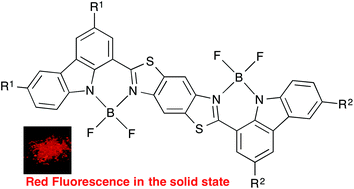
Carbazole-based BODIPY dimers 2a–g were synthesized via direct arylation. They showed red-shifted solid-state fluorescence spectra as compared with the corresponding monomer. In addition, unsymmetrical dimers 2d, 2f, and 2g with two different substituents showed red fluorescence with improved quantum yields in the solid state.


 Please wait while we load your content...
Please wait while we load your content...