Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
[18F]Fluoroalkyl azides for rapid radiolabeling and (Re)investigation of their potential towards in vivo click chemistry†
Christoph
Denk
a,
Martin
Wilkovitsch
a,
Philipp
Skrinjar
a,
Dennis
Svatunek
a,
Severin
Mairinger
b,
Claudia
Kuntner
b,
Thomas
Filip
b,
Johannes
Fröhlich
a,
Thomas
Wanek
b and
Hannes
Mikula
 *a
*a
aInstitute of Applied Synthetic Chemistry, Vienna University of Technology (TU Wien), Austria. E-mail: hannes.mikula@tuwien.ac.at
bHealth and Environment Department, Biomedical Systems, Austrian Institute of Technology (AIT), Seibersdorf, Austria
First published on 27th June 2017
Abstract
In recent years, radiofluorinated alkyl azides have been reported for click radiolabeling and pretargeted PET imaging, but only little is known about the biodistribution and metabolism of these compounds. In this work, we present a significantly improved procedure for the synthesis of [18F]fluoroethyl azide and reinvestigated this radiolabeled probe in detail showing poor stability and very restricted suitability for in vivo application. Therefore, modified low-molecular-weight [18F]fluoroalkyl azides were developed. Propargyl-tagged endomorphin-1 (as model compound) was successfully radiolabeled in high yield and short reaction time making these probes useful and efficient bioorthogonal tools for rapid radiolabeling. Biodistribution, pharmacokinetics and in vivo stability were studied by preclinical PET/MR scanning and metabolite analysis. The results of this study revealed only limited applicability of [18F]fluoroalkyl azides for in vivo application.
Introduction
Positron emission tomography (PET) allows non-invasive molecular imaging and quantification of metabolic processes. High sensitivity, spatial and temporal resolution made PET an indispensable diagnostic tool in the clinic and in biomedical research. The versatility of this imaging modality is highly dependent on the availability of specifically radiolabeled probes. Short-lived nuclides are generally favored due to low patient radiation doses and high specific activities.1 However, sufficient time is still necessary for production, quality control, delivery and application of radiolabeled probes. Fluorine-18 (half-life of 109.8 min) is considered to be the ideal PET radioisotope enabling the radiochemist to conduct multi-step synthesis and purifications using chromatographic methods.2–4[18F]Fluoroethyl azide ([18F]1-azido-2-fluoroethane, [18F]FEA) is reported to be a highly versatile tool for PET imaging and radiochemistry, and has frequently been used as a prosthetic group for rapid radiolabeling of precursor compounds modified with terminal alkynes through copper-catalyzed alkyne azide cycloadditions (CuAAC).5–7 These reactions are fast, highly selective, high yielding and – in contrast to direct 18F-labeling – can be carried out under mild reaction conditions without the need of protecting groups. Hence, this kind of bioorthogonal ligation is a very useful tool for the radiolabeling of structurally complex compounds like peptides and other biomolecules (Fig. 1a).5,6,8–12
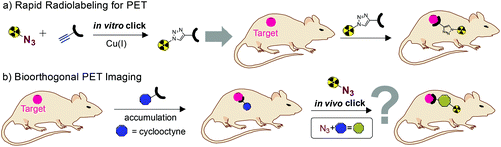 | ||
| Fig. 1 Radiolabeled alkyl azides as (a) prosthetic groups for rapid radiolabeling (in vitro click) and (b) secondary imaging agents for pretargeted bioorthogonal PET imaging (in vivo click). | ||
Furthermore, [18F]FEA has been proposed as a promising secondary agent for pretargeted PET imaging applying in vivo bioorthogonal ligation to cyclooctyne-tagged probes (e.g. antibodies).11 This two-step strategy is supposed to enable imaging of long circulating nanomedicines with short half-life nuclides such as fluorine-18. First, the probe modified with a reactive tag (reporter) is administered and allowed to accumulate in target tissue. After clearance of excess probe a radiolabeled click imaging agent is given that is supposed to react with the pre-administered reporter in vivo at the target site (Fig. 1b). The strain promoted alkyne–azide cycloaddition (SPAAC) using organic azides and cyclooctynes as reactants has frequently been applied in vitro and in vivo.13–17
Moreover, promising results on pretargeted PET applying pre-administered cyclooctyne-labeled nanoparticles followed by administration of an azide-functionalized fluorine-18-labeled mini-PEG have been reported.13 Nevertheless, this highly polar radiolabeled probe is unlikely to cross cell membranes and thus is not suitable in combination with internalizing chemical reporters. Hence, a metabolically stable probe with fast pharmacokinetics and medium polarity (cell permeability) is supposed to be of high value for SPAAC-mediated in vivo applications.
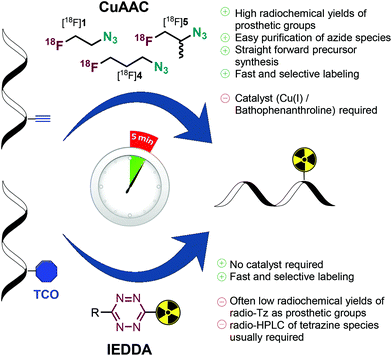
In recent years, the inverse electron demand Diels–Alder (IEDDA) reactions between 1,2,4,5-tetrazines and trans-cyclooctenes (TCOs) has successfully been applied in rapid radiolabeling and bioorthogonal PET imaging.7,18–23 Rate constants of this ligation are particularly high, however, in addition to increased steric demand, tetrazine (radio)chemistry does not meet the robustness of low-molecular-weight [18F]fluoroalkyl azides.
Despite the applicability of [18F]FEA several problems are associated with this low-molecular weight agent. [18F]FEA is highly volatile and usually purified from the labeling reaction mixture by co-distillation with acetonitrile.5 However this purification is time-consuming and care must be taken not to exceed a distillation temperature of 90 °C. Otherwise, the precursor is carried over causing impurities and interferences in subsequent cycloaddition reactions.24 Moreover, the tosylated precursor is highly viscous making it difficult to handle in small quantities. Stable 2-fluoroethyl azide required for synthesis of cold reference materials is potentially explosive, gaseous and difficult to handle in isolated form.5
In this paper we aim to shed light on the applicability of [18F]FEA and related compounds as click imaging agents by detailed investigation of biodistribution and metabolism. Furthermore, we present new tools that circumvent problems associated with [18F]FEA. A novel crystalline precursor for efficient radiolabeling is presented enabling the synthesis of [18F]FEA in high yields also avoiding problems related to distillation carry-over. Moreover, two new low-molecular-weight [18F]fluoroalkyl azides (1-azido-3-[18F]fluoropropane and 2-azido-1-[18F]fluoropropane) are presented as prosthetic groups with beneficial properties, and further investigated with emphasis on applicability as click imaging agents for pretargeted PET imaging showing only very limited applicability.
Results and discussion
Radiosynthesis and rapid radiolabeling
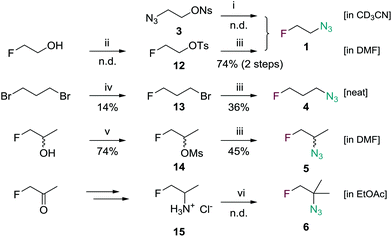
Radiosynthesis of [18F]1 is commonly carried out using K2CO3/Kryptofix-222 (K222)-mediated nucleophilic radiofluorination of the tosylated precursor 2-azidoethyl 4-methylbenzenesulfonate (2).5,25,26 After short reaction time at elevated temperature (80–110 °C) the product is isolated by co-distillation with acetonitrile. However, time-consuming distillation at low temperature, reduced pressure and the use of low precursor mass are required to minimize carry-over of the volatile tosylate 2. Co-distilled precursor causes chemical impurities and furthermore competes with [18F]1 within the cycloaddition with alkyne groups leading to low yields in the ligation reaction.
To circumvent this problem 2-azidoethyl 4-nitrobenzenesulfonate (3) was synthesized as novel precursor material from 2-azidoethanol and nosyl chloride in 67% yield (Fig. 2). The nosylate was obtained as crystalline solid, thus making handling of small quantities significantly easier compared to the highly viscous, commonly used precursor 2-azidoethyl 4-methylbenzenesulfonate (2). [18F]1 was obtained by reacting 3 with azeotropically dried [18F]fluoride and K2CO3/K222 in acetonitrile for 5 min at 105 °C (Scheme 1). Time-efficient distillation at 105–110 °C in a stream of argon afforded [18F]1 in 75% decay-corrected yield and >99% radiochemical purity. Most noteworthy, no precursor was detectable in the distilled product by HPLC due to reduced volatility of 3.
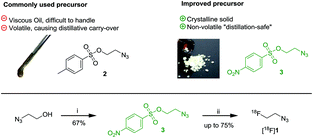 | ||
| Fig. 2 Advantages (in comparison to the commonly used precursor 2) and synthesis of precursor 3, and subsequent radiolabeling affording [18F]1. [(i) NsCl, N(Et)3; (ii) 18F-HF, K2CO3, Kryptofix-222]. | ||
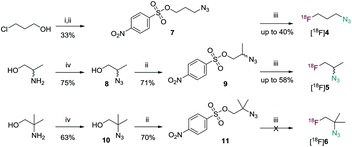 | ||
| Scheme 1 Synthesis of precursor materials 7, 9, 11 and subsequent radiolabeling. [(i) NaN3; (ii) NsCl, NEt3; (iii) [18F]HF, K2CO3, Kryptofix-222; (iv) 1H-imidazole-1-sulfonyl azide, K2CO3, CuSO4]. | ||
Due to the toxicity associated with acetonitrile a different method for purification was applied to obtain [18F]1 for in vivo investigations. Precursor 3 was radiolabeled using an automated GE Tracerlab FX module with an integrated HPLC purification system. A water/ethanol gradient in combination with a reversed phase column was used to yield [18F]1 in biocompatible media in 48% decay-corrected radiochemical yield (non-optimized) with a radiochemical purity of 99.6%. Identity of [18F]1 was confirmed by HPLC, and stability of the formulation was assessed after 6 h still revealing >97% purity.
However, our findings from the murine metabolic study of [18F]1 showed high bone uptake indicating low metabolic stability and release of free 18F− (for details see in vivo section). In contrast, probes prepared by rapid radiolabeling using [18F]1 are known to exhibit high metabolic stability in vivo.27 Therefore, we assume an influence of the azide functionality on defluorination. To minimize in vivo degradation of [18F]fluoroalkyl azides, we have considered [18F]4, [18F]5 and [18F]6 (Scheme 1) as structurally similar analogs to [18F]1.
We assumed that [18F]1-azido-3-fluoropropane ([18F]4) might show reduced tendency to release fluoride due to decreased electronic interactions between the azide functionality and 18F. Furthermore, [18F]2-azido-1-fluoropropane ([18F]5) and [18F]2-azido-2-methyl-1-fluoropropane ([18F]6) were considered to investigate the influence of increased steric hindrance on in vivo defluorination.
3-Azidopropyl 4-nitrobenzenesulfonate (7) was prepared as precursor compound for [18F]4 in two steps starting from 3-chloropropan-1-ol. The crude product was treated with p-nitrobenzenesulfonyl chloride and triethylamine to afford 7 as a crystalline solid in 33% overall yield (Scheme 1). Radiolabeling using conditions described above for the synthesis of [18F]1 afforded [18F]4 after co-distillation with acetonitrile in 39.3% decay corrected radiochemical yield and with a radiochemical purity of >98%. Preparative HPLC was used instead of distillation to obtain pure product (without acetonitrile) for in vivo investigation. Identity of [18F]4 was confirmed by comparison via HPLC with an authentic standard, and stability of the formulation was assessed by radio-HPLC three hours post radiosynthesis still revealing >89% radiochemical purity.
[18F]5 was synthesized from crystalline precursor 9. Commercially available racemic alaninol was subjected to copper-catalyzed diazotransfer conditions using 1H-imidazole-1-sulfonyl azide to obtain 2-azidopropan-1-ol (8) in 75% yield.28,29 Nosylation of 8 gave 9 as a racemic mixture in 71% yield (Scheme 1). Noteworthy, alaninol is commercially available in optically pure form (R or S) making enantiopure [18F]5 accessible following the presented procedure. However, in this study racemic [18F]5 was further investigated. 9 was then reacted with azeotropically dried 18F-K222 complex in anhydrous acetonitrile for 7 min at 100 °C followed by distillation in a stream of argon to obtain [18F]5 as solution in anhydrous acetonitrile with 58% decay-corrected yield and a radiochemical purity of 99.7%. For in vivo studies acetonitrile-free [18F]5 was synthesized on an automated GE Tracerlab FX module followed by preparative HPLC purification (27% decay-corrected radiochemical yield, >96% radiochemical purity).
Applying a similar synthetic strategy towards [18F]6, 2-amino-2-methylpropan-1-ol was subjected to copper-catalyzed diazotransfer conditions to obtain 2-azido-2-methylpropanol (10). The nosylated compound 11 was obtained in 70% yield by reaction of alcohol 10 with nosyl chloride and triethylamine (Scheme 1). Unfortunately, radiolabeling attempts using the K2CO3/K222 method resulted in formation of an unknown radiolabeled species of unexpected lipophilicity. Its HPLC retention time did not match the retention time of an authentic standard. Although the identity of the formed compound could not be clarified, modeling the labeling reaction in deuterated acetonitrile using (“cold”) potassium fluoride followed by 19F-NMR spectroscopy indicated the absence of a primary alkyl fluoride (chemical shift in the range of −220 ppm). Instead signals at −105 ppm indicated the formation of undesired products due to elimination and/or rearrangement.
Access to non-radioactive (“cold”) reference compounds is essential for identification of the radiolabeled agent by HPLC. 1-Azido-2-fluoroethane (1) is highly volatile and potentially explosive and should thus not be isolated and handled in pure form.5 The previously reported30 tosylation of 2-fluoroethan-1-ol followed by reaction with sodium azide and subsequent filtration yielded a 1.36 M solution of 1 in DMF that was used successfully for cycloaddition to alkynes. Furthermore, DMF-free 1 was obtained by reacting 3 with anhydrous potassium fluoride and Kryptofix-222 in acetonitrile followed by evaporation at low pressure and trapping of volatiles in a liquid nitrogen trap (Scheme 2).
1-Azido-3-fluoropropane (4) was synthesized starting from 1,3-dibromopropane that was converted to 1-bromo-3-fluoropropane (13). Although 13 was isolated in only 14% yield its synthesis from cheap starting materials is still economically favorable.31 Nucleophilic substitution with sodium azide afforded 3 in 36% yield. Due to a higher boiling point compared to 1 we were able to isolate neat 3 as colorless liquid by careful distillation; a clear benefit in case subsequent chemical modification is intended. Although we did not encounter any problems, safety precautions such as blast shields and performing the reaction in small scale are highly recommended. Maximum scale in this study was less than 1 g. Moreover, all reactions involving azide as a nucleophile must be free of alkyl halides including dichloromethane. We observed the formation of potentially dangerous (explosive) diazidomethane when traces of dichloromethane were present in the reaction mixture. 2-Azido-1-fluoropropane (5) was also obtained as a solution in DMF (1.05 M) by mesylation of commercially available 1-fluoropropan-2-ol followed by reaction with sodium azide in anhydrous DMF.32 No attempts were made to isolate neat 5. The tertiary azide 6 was obtained as a solution in ethyl acetate after applying diazotransfer conditions to 2-amino-2-methyl-1-fluoropropane hydrochloride (15),29 which was prepared following a known procedure.33
HPLC retention times of radiolabeled species (in acetonitrile for rapid radiolabeling or in aqueous medium for in vivo application) were compared with retention times of cold reference compounds to confirm the identity of radiolabeled agents (see ESI† for chromatographic data). Compounds that were not isolated (1, 5 and 6) were ligated with (1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-ylmethanol (BCN)34 as cyclooctyne in a strain-promoted alkyne–azide cycloaddition. The resulting triazoles 16, 17 and 18 were isolated and fully characterized by NMR and MS (Scheme 3). [18F]1 and [18F]5 were reacted with BCN and formation of the ligation products [18F]16 and [18F]17 was confirmed by comparison with their non-radioactive counterparts. Reacting the product afforded after radiolabeling of precursor 11 with BCN led to formation of an unknown compound not matching 18 and thus confirming the formation of an undesired re-arrangement or elimination product during the radiolabeling reaction of 11 (as mentioned above).
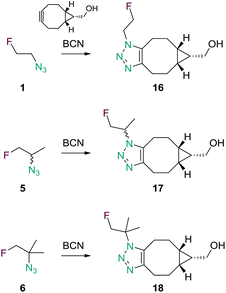 | ||
| Scheme 3 SPAAC reaction of 1, 5 and 6 with the cyclooctyne BCN yielding the ligation products 16, 17 and 18. | ||
Application of [18F]1 as prosthetic group for rapid “click” radiolabeling is well documented.5,6,8–10,12,26,35 Several modifications of the original procedure from Arstad and Glaser were reported to shorten reaction times and eliminate the need for heating. Noteworthy, addition of the water-soluble copper–ligand bathophenanthroline sodium sulfonate reduces the required amount of copper and sodium ascorbate while maintaining or even enhancing ligation rates at room temperature.6
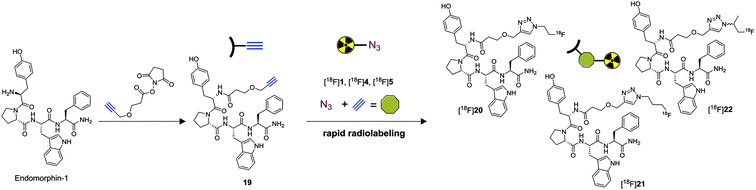
To test and compare [18F]4 and [18F]5 as prosthetic groups against already established [18F]1 we have selected the tetrapeptide endomorphin-1 (EM-1) as substrate. EM-1 is an endogenous substance and powerful μ-opioid receptor agonist (Ki = 360 pM) that is of potential interest for imaging purposes.36 EM-1 is commercially available and possesses only one nucleophilic amine to allow for site specific modification. EM-1 was treated with commercially available 2,5-dioxopyrrolidin-1-yl-3-(prop-2-yn-1-yloxy)propanoate and triethylamine to introduce an terminal alkyne (Scheme 4). Propargyl endomorphin-1 19 was obtained in 42% yield after purification by HPLC. Ligation products 20, 21 and 22 were obtained by reaction of 19 with 1, 4 and 5 using the CuSO4/sodium ascorbate/bathophenantroline procedure, and isolated by RP-HPLC. [18F]20, [18F]21 were obtained in good radiochemical yields within 10 minutes by reacting 1 mg of 19 with [18F]1 or [18F]4 using the same procedure (Scheme 4).
As [18F]5 was prepared as a racemic mixture, it needs to be mentioned that radiolabeling of chiral substances produces a diastereomeric mixture potentially complicating analysis. In case of required optically pure material enantiopure (2S)-1-azido-2-propanol37 can be used for the synthesis of precursor 9. Subjecting [18F]5 to similar experimental conditions yielded [18F]22 as an inseparable mixture of diastereomers in excellent radiochemical yield of 98%. Retention times of the ligation products were compared by radio-HPLC with authentic reference compounds (20–22). In addition, the plasma stability of all ligation products was determined by incubation in human blood plasma for 2 hours followed by radio-TLC analysis. Similar stabilities (≥80%) were observed for all ligation products further indicating the applicability of [18F]4 and [18F]5 as prosthetic groups for rapid radiolabeling.
In vivo investigations
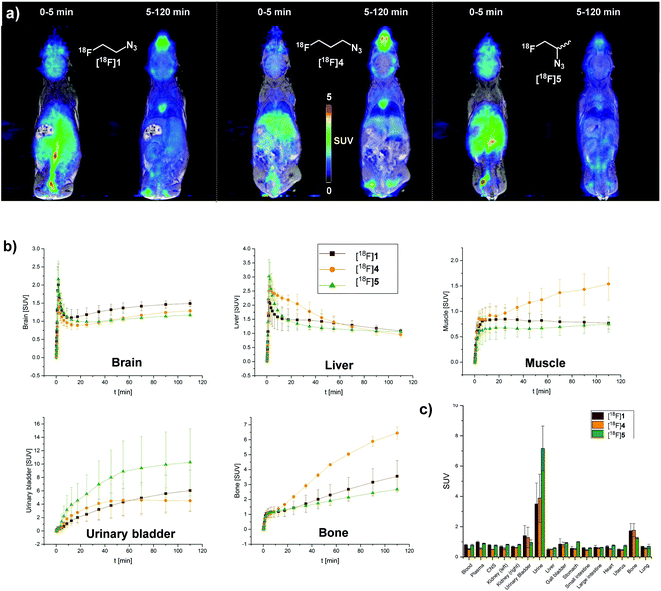
[18F]1, [18F]4, and [18F]5 were injected into female BALB/c mice and dynamic PET (n = 4 for [18F]1, n = 2 for [18F]4 and [18F]5) or PET/MR (n = 1 for all test substances) scanning was performed for 2 hours. Coronal PET/MR images are shown in Fig. 3a. Analysis of organ time activity curves (Fig. 3b) reveals a uniform uptake of all agents in most analyzed organs that slowly decreases from ∼1.5 to ∼1 SUV (standardized uptake value) during the course of the experiment. High uptake in the urinary bladder suggests renal elimination as main excretion route for all investigated substances. The secondary azide [18F]5 exhibits the fastest clearance, a clear benefit when application as click imaging agent in bioorthogonal PET imaging is intended. Interestingly, brain uptake shows a slight but steady increase for all analyzed tracers.[18F]4 exhibits a higher muscular uptake compared to [18F]1 and [18F]5 that increased throughout the experiment (Fig. 3b). Most noteworthy, an increase of radioactivity concentration in bone was observed – a good indicator for metabolic instability and in vivo defluorination of the tracer molecules. Activity concentration in bone peaked at 3.6 SUV for [18F]1 and 6.5 SUV for [18F]4, thus showing that in vivo stability could not be improved by insertion of one extra methylene group. [18F]5 showed lowest bone uptake (peak SUV = 2.7) compared to [18F]1 and [18F]4 indicating improved in vivo stability over [18F]1 and [18F]4 (Fig. 3b). In addition, coronal PET/MR images (Fig. 3a) show skull uptake in the 5–120 min summation images, which is least pronounced for [18F]5 while [18F]4 exhibits the highest uptake. Placing a “total box” ROI over the entire animal revealed constant activity over the entire imaging period showing that [18F]1, [18F]4 and [18F]5 are not excreted by respiration.
Following imaging, blood and urine samples were collected and the mice were sacrificed. Organ samples were collected and measured for radioactivity in a gamma counter. Data obtained at 120 min post injection of the tracers match the results of the PET experiment (Fig. 3c). Murine plasma was obtained by centrifugation of whole blood in heparin tubes, and plasma proteins were precipitated by addition of an equal volume of acetonitrile. Following centrifugation, the supernatant was injected into the HPLC system for analysis of plasma metabolites. Due to the volatile nature of the test substances radio-HPLC was carried out instead of radio-TLC. Urine samples were injected after dilution with water. HPLC data shows diminutive (<4% of total radioactivity) amounts of intact compounds while the main fraction of radioactivity (>90%) was detected as highly polar compound, most likely free [18F]fluoride ions confirming the metabolic instability of [18F]1, [18F]4 and [18F]5. Considering the rate of bone uptake (Fig. 2b) [18F]5 shows increased stability compared to [18F]1 and [18F]4. This data is also reflected by plasma metabolite HPLC analysis, wherein [18F]4 displayed the highest percentage of intact tracer 120 min post administration. Despite nuances in metabolic stability, degradation seems to happen on a time scale that is not compatible with reaction kinetics of the SPAAC ligation. In addition, high bone uptake will contribute to background signal hindering image interpretation. Hence, [18F]fluoroalkyl azides do not represent suitable click imaging agents for in vivo application.
Although our findings and conclusion regarding the in vivo applicability of [18F]1 deviate from previously published results,11 it needs to be mentioned that time activity curves, biodistribution data, and assessment of in vivo stability of [18F]1 have not been shown so far. In general, click imaging agents for pretargeted PET imaging need to show good metabolic stability to allow for sufficient reaction and diffusion time in target tissue. This is of particular importance for chemistries with only slow to intermediate reaction kinetics such as SPAAC. Furthermore, metabolic degradation products contribute to background signal, and might show specific uptake (e.g. [18F]fluoride in bone), thus complicating image interpretation. In this regard, tetrazine ligations are preferred and advantageous over SPAAC.38
Conclusion
In summary, we present an improved synthesis of [18F]fluoroethyl azide based on a novel precursor substance that is crystalline, easier to handle, and less volatile thus preventing the problem of distillative carry-over. In addition, two novel [18F]fluoroalkyl azide agents ([18F]4, [18F]5) were developed expanding the library of prosthetic groups for 18F-labeling. Rapid radiolabeling was demonstrated on endogenous μ-opioid agonistic peptide endomorphin-1. The labeling was successfully carried out in short time (5–10 min) achieving high radiochemical yields. Despite very limited applicability for in vivo reaction our [18F]fluoroalkyl azides were shown to be useful and efficient alternatives for rapid radiolabeling compared to tetrazine-based agents (Fig. 4).Acknowledgements
The authors acknowledge the TU Wien University Library for financial support through its Open Access Funding Program.References
- D. L. Bailey, D. W. Townsend, M. N. Maisey and P. E. Valk, Positron emission tomography: basic sciences, Springer, Secaucus, NJ, 2005, ISBN 1852337982 Search PubMed.
- R. N. Bryan, Introduction to the science of medical imaging, Cambridge University Press, 2010, ISBN 9780521747622 Search PubMed.
- O. Jacobson, D. O. Kiesewetter and X. Chen, Bioconjugate Chem., 2015, 26, 1–18 CrossRef CAS PubMed.
- J.-L. Zeng, J. Wang and J.-A. Ma, Bioconjugate Chem., 2015, 26, 1000–1003 CrossRef CAS PubMed.
- M. Glaser and E. Arstad, Bioconjugate Chem., 2007, 18, 989–993 CrossRef CAS PubMed.
- D. Zhou, W. Chu, C. S. Dence, R. H. Mach and M. J. Welch, Nucl. Med. Biol., 2012, 39, 1175–1181 CrossRef CAS PubMed.
- J.-P. Meyer, P. Adumeau, J. S. Lewis and B. M. Zeglis, Bioconjugate Chem., 2016, 27, 298–301 CrossRef CAS PubMed.
- R. Bejot, L. Carroll, K. Bhakoo, J. Declerck and V. Gouverneur, Bioorg. Med. Chem., 2012, 20, 324–329 CrossRef CAS PubMed.
- M. Glaser, M. Solbakken, D. R. Turton, R. Pettitt, J. Barnett, J. Arukwe, H. Karlsen, A. Cuthbertson, S. K. Luthra and E. Årstad, Amino Acids, 2009, 37, 717–724 CrossRef CAS PubMed.
- L. Jia, Z. Cheng, L. Shi, J. Li, C. Wang, D. Jiang, W. Zhou, H. Meng, Y. Qi, D. Cheng and L. Zhang, Appl. Radiat. Isot., 2013, 75, 64–70 CrossRef CAS PubMed.
- H. L. Evans, R. L. Slade, L. Carroll, G. Smith, Q.-D. Nguyen, L. Iddon, N. N. Kamaly, H. Stöckmann, F. J. Leeper, E. O. Aboagye, A. C. Spivey and O. Aboagye, Chem. Commun., 2012, 48, 991–993 RSC.
- K. Kettenbach, H. Schieferstein, T. L. Ross, K. Kettenbach, H. Schieferstein and T. L. Ross, Biomed Res. Int., 2014, 2014, 1–16 CrossRef PubMed.
- S. B. Lee, H. L. Kim, H.-J. Jeong, S. T. Lim, M.-H. Sohn and D. W. Kim, Angew. Chem., Int. Ed., 2013, 52, 10549–10552 CrossRef CAS PubMed.
- J. A. Prescher and C. R. Bertozzi, Nat. Chem. Biol., 2005, 1, 13–21 CrossRef CAS PubMed.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef CAS PubMed.
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS PubMed.
- T. Plass, S. Milles, C. Koehler, C. Schultz and E. A. Lemke, Angew. Chem., Int. Ed., 2011, 50, 3878–3881 CrossRef CAS PubMed.
- R. Rossin, T. Läppchen, S. M. Van Den Bosch, R. Laforest and M. S. Robillard, J. Nucl. Med., 2013, 54, 1989–1995 CrossRef CAS PubMed.
- R. Rossin, P. R. Verkerk, S. M. Van Den Bosch, R. C. M. Vulders, I. Verel, J. Lub and M. S. Robillard, Angew. Chem., Int. Ed., 2010, 49, 3375–3378 CrossRef CAS PubMed.
- R. Rossin and M. S. Robillard, Curr. Opin. Chem. Biol., 2014, 21, 161–169 CrossRef CAS PubMed.
- J. P. Meyer, J. L. Houghton, P. Kozlowski, D. Abdel-Atti, T. Reiner, N. V. K. Pillarsetty, W. W. Scholz, B. M. Zeglis and J. S. Lewis, Bioconjugate Chem., 2016, 27, 298–301 CrossRef CAS PubMed.
- B. M. Zeglis, K. K. Sevak, T. Reiner, P. Mohindra, S. D. Carlin, P. Zanzonico, R. Weissleder and J. S. Lewis, J. Nucl. Med., 2013, 1389–1396 CrossRef CAS PubMed.
- N. K. Devaraj and R. Weissleder, Acc. Chem. Res., 2011, 44, 816–827 CrossRef CAS PubMed.
- M. Pretze, D. Pietzsch and C. Mamat, Molecules, 2013, 18, 8618–8665 CrossRef CAS PubMed.
- D. Kobus, Y. Giesen, R. Ullrich, H. Backes and B. Neumaier, Appl. Radiat. Isot., 2009, 67, 1977–1984 CrossRef CAS PubMed.
- L. Iddon, J. Leyton, B. Indrevoll, M. Glaser, E. G. Robins, A. J. T. George, A. Cuthbertson, S. K. Luthra and E. O. Aboagye, Bioorg. Med. Chem. Lett., 2011, 21, 3122–3127 CrossRef CAS PubMed.
- G. Smith, M. Glaser, M. Perumal, Q.-D. Nguyen, B. Shan, E. Årstad and E. O. Aboagye, J. Med. Chem., 2008, 51, 8057–8067 CrossRef CAS PubMed.
- E. D. Goddard-Borger and R. V. Stick, Org. Lett., 2007, 9, 3797–3800 CrossRef CAS PubMed.
- E. D. Goddard-Borger and R. V. Stick, Org. Lett., 2011, 13, 2514–2514 CrossRef CAS.
- J. Li, L. Shi, L. Jia, D. Jiang, W. Zhou, W. Hu, Y. Qi and L. Zhang, Bioorg. Med. Chem., 2012, 20, 3850–3855 CrossRef CAS PubMed.
- F. W. Hoffmann, J. Org. Chem., 1949, 14, 105–110 CrossRef CAS.
- C. Jean-Damien, M. Haley and D. Binch, WO2011143426A1, 2011 Search PubMed.
- D. Ok, M. H. Fisher, M. J. Wyvratt and P. T. Meinke, Tetrahedron Lett., 1999, 40, 3831–3834 CrossRef CAS.
- J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. Van Hest, D. J. Lefeber, P. Friedl and F. L. Van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422–9425 CrossRef CAS PubMed.
- A. Gaeta, J. Woodcraft, S. Plant, J. Goggi, P. Jones, M. Battle, W. Trigg, S. K. Luthra and M. Glaser, Bioorg. Med. Chem. Lett., 2010, 20, 4649–4652 CrossRef CAS PubMed.
- G. Horvath, Pharmacol. Ther., 2000, 88, 437–463 CrossRef CAS PubMed.
- S.-W. Chen, S. S. Thakur, W. Li, C.-K. Shin, R. B. Kawthekar and G.-J. Kim, J. Mol. Catal. A: Chem., 2006, 259, 116–120 CrossRef CAS.
- J. C. Knight and B. Cornelissen, Am. J. Nucl. Med. Mol. Imaging, 2014, 4, 96–113 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ob00880e |
| This journal is © The Royal Society of Chemistry 2017 |