Cationic polycyclization of ynamides: building up molecular complexity†
Abstract
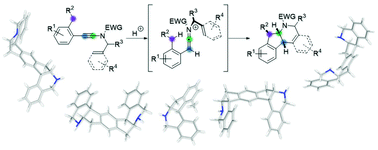
Polycyclization reactions are among the most efficient synthetic tools for the synthesis of complex, polycyclic molecules in a single operation from simple starting materials. We report in this manuscript a full account on the discovery and development of a novel cationic polycyclization from readily available ynamides. Simple activation of these building blocks under acidic conditions enables the generation of highly reactive activated keteniminium ions, which triggers an unprecedented cationic polycyclization yielding highly substituted polycyclic nitrogen heterocycles possessing up to seven fused cycles and three contiguous stereocenters.
- This article is part of the themed collection: Polycyclic Reactions in Synthesis and Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...