Open Access Article
Open Access ArticleGraphene oxide inhibits malaria parasite invasion and delays parasitic growth in vitro†
Kenry‡
 abc,
Ying Bena
Lim‡
cd,
Mui Hoon
Nai
e,
Jianshu
Cao
df,
Kian Ping
Loh
abc,
Ying Bena
Lim‡
cd,
Mui Hoon
Nai
e,
Jianshu
Cao
df,
Kian Ping
Loh
 abg and
Chwee Teck
Lim
abg and
Chwee Teck
Lim
 *abcde
*abcde
aNUS Graduate School for Integrative Sciences and Engineering, National University of Singapore, Singapore 117456
bCentre for Advanced 2D Materials and Graphene Research Centre, National University of Singapore, Singapore 117543
cDepartment of Biomedical Engineering, National University of Singapore, Singapore 117576. E-mail: ctlim@nus.edu.sg
dSingapore-MIT Alliance for Research and Technology (SMART) Centre, Infectious Diseases IRG, Singapore 138602
eMechanobiology Institute, National University of Singapore, Singapore 117411
fDepartment of Chemistry, Massachusetts Institute of Technology, Cambridge, USA 02139
gDepartment of Chemistry, National University of Singapore, Singapore 117543
First published on 28th August 2017
Abstract
The interactions between graphene oxide (GO) and various biological entities have been actively investigated in recent years, resulting in numerous potential bioapplications of these nanomaterials. Despite this, the biological interactions between GO and disease-causing protozoan parasites have not been well elucidated and remain relatively unexplored. Here, we investigate the in vitro interactions between GO nanosheets and a particular species of malaria parasites, Plasmodium falciparum (P. falciparum). We hypothesize that GO nanosheets may exhibit antimalarial characteristic via action mechanisms of physical obstruction of P. falciparum parasites as well as nutrient depletion. To ascertain this, we characterize the physical interactions between GO nanosheets, red blood cells (RBCs), and malarial parasites as well as the adsorption of several biomolecules necessary for parasitic survival and growth on GO nanosheets. Subsequent to establishing the origin of this antimalarial behavior of GO nanosheets, their efficiency in inhibiting parasite invasion is evaluated. We observe that GO nanosheets at various tested concentrations significantly inhibit the invasion of malaria parasites into RBCs. Furthermore, GO nanosheets delay parasite progression from the ring to the trophozoite stage. Overall, this study may further shed light on the graphene-parasite interactions and potentially facilitate the development of nanomaterial-based strategies for combating malaria.
1. Introduction
Graphene oxide (GO) is the oxygenated derivative of two-dimensional (2D) graphene.1,2 Among the numerous 2D nanomaterials,3–6 GO stands out due to its large surface area, unique bio-physico-chemical properties, and excellent in vitro and in vivo biocompatibility.7 Therefore, it has been actively explored for a wide variety of biological and biomedical applications. These include biological sensing,8,9 cellular imaging,10,11 cell culture,12–15 antibacterial16,17 and antithrombotic18 coatings, and cancer therapeutics.19,20 Interestingly, these bioapplications hinge significantly on the molecular and cellular interactions between GO and various biological entities.GO possesses a unique 2D structural feature which endows it a large surface area as a template for cargo loading.21–23 It is a versatile nanomaterial whose basal plane and periphery are decorated with various oxygen-containing groups. In fact, their presence on the surface of GO enhances its dispersibility, solubility, and stability in aqueous media and facilitates its chemical functionalization.1,2 At the same time, these hydrophilic functionalities and aromatic domains enable GO to interact specifically with a plethora of biomolecules, such as proteins, peptides, and amino acids.24,25 In addition, they serve as pre-concentration platforms for biomolecules through electrostatic interaction or π–π stacking.12,13,18 Intriguingly, GO is also known to display excellent biocompatibility and low toxicity.3 It has been reported that GO possesses a lower cytotoxicity than carbon nanotubes.26,27 Moreover, contrary to the hydrophobic pristine graphene, the hydrophilic GO can be taken up by cells with minimal toxic effects.26,28 Importantly, GO has been demonstrated to induce negligible in vivo toxicity in animals at low and medium concentrations.26,29 All these bio-physico-chemical features render the versatile GO highly advantageous for bioapplications, as compared to other carbon-based and 2D nanomaterials.30,31 In particular, the exceptional biomolecule loading capability of GO coupled with its large surface area and excellent biocompatibility may potentially be exploited to obstruct the occurrence of specific biological events, such as cellular and/or parasitic growth, which form the basis of many physiological diseases.
Of all parasitic diseases, malaria remains one of the world's most prevalent and deadly infectious diseases and a global public health concern.32,33 In 2013 alone, there were about 200 million clinical cases, with approximately 584![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths worldwide.34 This disease is caused by the protozoan parasites of the genus Plasmodium, which have a complex life cycle alternating between sexual and asexual reproduction in invertebrate mosquitoes and vertebrate hosts, respectively. In fact, the asexual malaria parasite infection in the peripheral blood circulation is the major cause behind the malarial disease pathology.35 Among the Plasmodium parasite species responsible for human malaria infections, Plasmodium falciparum (P. falciparum) causes the highest rates of mortality and morbidity.35,36P. falciparum malaria parasites develop in two different hosts, i.e., female Anopheles mosquito and human. In human, the malaria parasites taking the form of sporozoites enter the bloodstream and travel towards liver to start their multiplication. Over the course of several days, tens of thousands of merozoites will be produced and released into the bloodstream to invade circulating red blood cells (RBCs). Subsequently, throughout their 44–48 h asexual cycle within these RBCs, these malaria parasites mature and undergo different stages of development, i.e., ring, trophozoite, and schizont, before the infected RBCs (iRBCs) burst and release more merozoites into the circulatory system. This part of the Plasmodium life cycle will repeat as merozoites invade even more healthy circulating RBCs.37
000 deaths worldwide.34 This disease is caused by the protozoan parasites of the genus Plasmodium, which have a complex life cycle alternating between sexual and asexual reproduction in invertebrate mosquitoes and vertebrate hosts, respectively. In fact, the asexual malaria parasite infection in the peripheral blood circulation is the major cause behind the malarial disease pathology.35 Among the Plasmodium parasite species responsible for human malaria infections, Plasmodium falciparum (P. falciparum) causes the highest rates of mortality and morbidity.35,36P. falciparum malaria parasites develop in two different hosts, i.e., female Anopheles mosquito and human. In human, the malaria parasites taking the form of sporozoites enter the bloodstream and travel towards liver to start their multiplication. Over the course of several days, tens of thousands of merozoites will be produced and released into the bloodstream to invade circulating red blood cells (RBCs). Subsequently, throughout their 44–48 h asexual cycle within these RBCs, these malaria parasites mature and undergo different stages of development, i.e., ring, trophozoite, and schizont, before the infected RBCs (iRBCs) burst and release more merozoites into the circulatory system. This part of the Plasmodium life cycle will repeat as merozoites invade even more healthy circulating RBCs.37
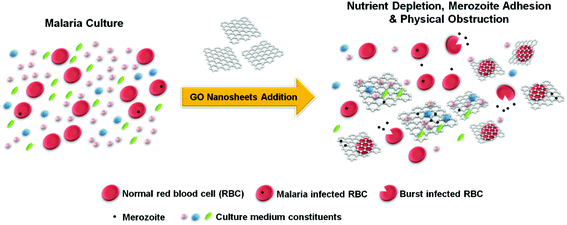
Here, using GO nanosheets (GOns) in aqueous suspension with distinct lateral size distributions and concentrations, we investigate the in vitro interactions between GOns and P. falciparum parasites. We hypothesize that due to their large specific surface area, GOns could play a key role in reducing malaria parasite invasion by physically trapping and obstructing the access of merozoites to RBCs. Furthermore, owing to their high loading capacity for biomolecules, these nanomaterials could reduce the availability of biomolecules necessary for parasite survival and invasion. Consequently, via these mechanisms, GOns are anticipated to exhibit antimalarial characteristic (Fig. 1).
2. Results and discussion
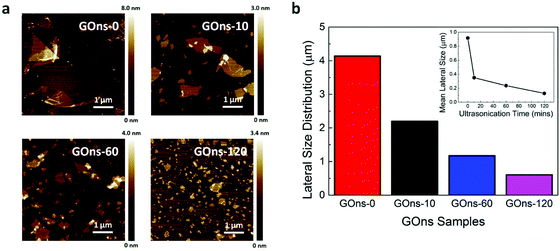
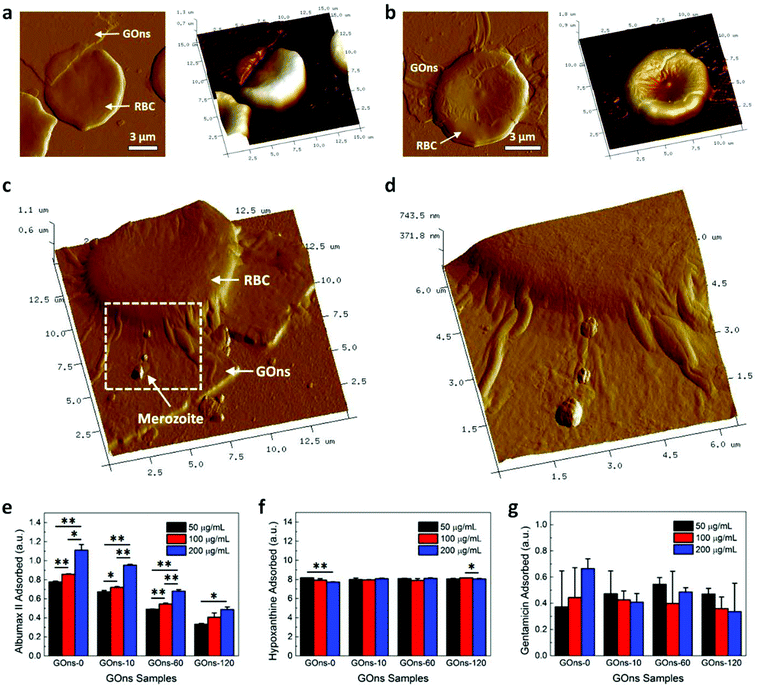
GOns in liquid suspension were first prepared using Hummer's method.18 As numerous studies have reported the effect of size of graphene nanomaterials in influencing their interactions with various biological moieties,38–40 we were also interested in characterizing this effect, specifically in affecting the possible antimalarial property of GOns. In yielding GOns with various lateral size distributions and mean lateral sizes, the as-prepared GOns (i.e., GOns-0) were subsequently subjected to ultrasonication treatment for durations of 10, 60, and 120 min (i.e., GOns-10, GOns-60, and GOns-120, respectively) (Fig. 2a), similar to that prepared in our previous work.24 It is worth noting that GOns with distinct lateral size distributions were prepared using ultrasonication as the surface chemical properties of GOns would be minimally compromised.16 Since GOns have irregular shapes, we defined the lateral size as the longest distance across a single nanosheet, similar to that described in reference.41 Next, to acquire the statistical data on the lateral size distributions (Fig. S1a–d† and Fig. 2b) and mean lateral sizes (inset of Fig. 2b) of the different GOns samples, we examined approximately 400 nanosheets. In light of the experimental data, it was evident that the as-prepared GOns possessed distinct lateral size distributions and mean lateral sizes.We next conjected that GOns with their large 2D surface area may serve as physical barriers that limit the access of merozoites to RBCs. This may be achieved through the direct physical attraction and trapping of merozoites onto the surface of GOns. To gain insights into the role of GOns in restricting merozoite accessibility, the physical interactions between GOns, RBCs, and merozoites were probed using AFM. Firstly, magnetically enriched late stage iRBCs were incubated with RBCs at 2.5% hematocrit and 5% parasitemia as well as 6.25 μg mL−1 GOns. A smear was then taken after a 12 h incubation when all iRBCs had burst and released their merozoites. The morphological shape and size distribution of the merozoites were next characterized. To ensure that merozoites were indeed the subject of characterization, the selected merozoites were those present around the ruptured iRBCs. We observed that these merozoites generally possessed ovoid bodies (Fig. S2a†) with an average length of 2.07 ± 0.86 μm (Fig. S2b†), similar to that reported in literature.42 We subsequently investigated the physical interactions between GOns and RBCs by probing the healthy RBCs in the presence of GOns, but in the absence of merozoites. Interestingly, we noted that at the tested concentration, GOns exhibited a high degree of cellular hemocompatibility in that RBC maintained its structural integrity and did not experience hemolysis as the sharp edge of GOns did not damage nor penetrate the RBC membrane (Fig. 3a). Instead, GOns were able to conform smoothly to the shape of RBC (Fig. 3b). These observations revealed the excellent hemocompatibility of GOns in relation to their interactions with RBCs. After establishing the nanomaterial hemocompatibility, the GOns-RBC-merozoite interactions were examined to investigate if GOns could indeed act as physical barriers in mitigating the malarial parasite invasion. Here, again, we observed that large GOns fully covered and conformed to the shape of RBC. At the same time, multiple merozoites released from burst schizonts adhered onto the surface of GOns (Fig. 3c, as indicated by the white arrow, and Fig. 3d). The adhesion of these merozoites on the GOns surface physically limited their access to the healthy RBCs and greatly hindered the parasite invasion.
The interactions between GOns and malaria parasites were further evaluated through Giemsa and fluorescence staining (Fig. S3 and S4,† respectively). Here, merozoites were observed to adhere directly on the larger GOns (Fig. S3†). This phenomenon suggests a dependency and direct correlation between the parasite adhesion and the surface area of GOns. To ascertain this observation, GOns were next drop-casted on glass coverslips and incubated with iRBCs. After the rupture of iRBCs, the surface of the parasite-incubated GOns was stained with ethidium bromide (EtBr) to visualize the cellular contents of iRBCs, including merozoites, on the GOns surface under a confocal microscope (Fig. S4†). Fluorescence corresponding to the EtBr-stained merozoites, as depicted by the clusters of the small dots surrounding parasite nuclei, was highly expressed on all GOns. This further shows that GOns were able to physically attract the merozoites.
Numerous studies have demonstrated the superior loading capacity of graphene nanomaterials for various serums, proteins, and amino acids, which is beneficial for a plethora of cellular and physiological functions.12,13,18 Depending on the nature of the interactions between proteins, cells, and graphene nanomaterials, the exceptional protein loading capacity of graphene nanomaterials may either enhance or hinder cellular development. The free amount of proteins in the system decreases as they get adsorbed on the nanomaterial surface. Consequently, cells which do not interact with the protein-loaded graphene nanomaterials may likely be deprived from the essential nutrients and growth factors, impeding cellular growth and proliferation. On this basis, we hypothesized that the hydrophilic GOns might possess a high loading capacity for various proteins, notably albumax II, in the malaria culture medium (MCM). This would lead to nutrient depletion for parasitic growth, resulting in the potential antimalarial behavior of GOns. To verify this, we evaluated the loading of individual MCM constituents on various GOns samples. Typically, MCM consists of tissue culture medium RPMI supplemented with serum protein albumax II, the purine source hypoxanthine, and the antibiotic gentamicin.43 Albumax II is serum albumin known to be lipid-carrying in blood and is essential for the optimal growth of malaria parasites.44 Hypoxanthine is a purine derivative necessary for the synthesis of nucleic acids and energy metabolism of the parasites as well as for the significant enhancement of their propagation.45 Gentamicin, meanwhile, serves as a water-soluble aminoglycoside bactericidal antibiotic in the complete culture medium.46 The relationship between the lateral size of GOns, their loading capacity for individual MCM constituents, and the potential antimalarial property of GOns were subsequently investigated.
We first measured the free absorbance of albumax II (Fig. S5a†), hypoxanthine (Fig. S5b†), and gentamicin (Fig. S5c†) prepared at various concentrations at the corresponding characteristic wavelengths of 280 nm, 260 nm, and 192 nm, respectively. A linear relationship was observed between the free absorbance of these MCM constituents and their concentrations (insets of Fig. S5a–c†). Next, we assessed the absorbance of albumax II, hypoxanthine, and gentamicin after incubating with GOns to evaluate the adsorption capacity of the individual MCM components on various GOns samples. The MCM constituents were fixed at a particular concentration and those of GOns were varied. After 48 h incubation, we observed a GOns concentration- and size-dependent adsorption of albumax II (Fig. 3e). As the concentration of GOns was increased from 50 μg mL−1 to 200 μg mL−1, the amount of adsorbed albumax II increased correspondingly. Interestingly, we noted that at particular GO concentrations, GOns-0 with the largest lateral size distribution and mean lateral size consistently exhibited the highest loading capacity for albumax II. The lowest adsorption was found with GOns-120 which has the smallest average size and size distribution. This observation implies a probable GOns size-dependent loading of albumax II on GOns. Also, more importantly, it highlights that the adsorption of albumax II was significantly dependent on the concentration of GOns. The GOns-albumax II association might be primarily driven by the hydrophobic interactions between GOns and the hydrophobic regions of albumax II, coupled with electrostatic interactions.47–49 Nonetheless, the GOns concentration-dependent loading capacity of biomolecules on GOns was not observed for both hypoxanthine and gentamicin (Fig. 3f and g), possibly due to their lower binding association and affinity to GOns. Several investigations have, in fact, revealed that the essential serum albumin serves as a critical source of nutrients for intraerythrocytic growth and cell cycle development of P. falciparum malaria parasites. It has also been emphasized that the intraerythrocytic parasites are able to uptake, breakdown, and degrade serum albumin, leading to a time-dependent proteolysis within the parasites.50 In addition, recent evidences have suggested that P. falciparum parasites respond negatively to starvations of proteins and amino acids, indicating their dependence on exogenous nutrients.51,52 Consequently, due to their exceptional loading capacity for MCM constituents, especially for serum albumin albumax II, GOns are anticipated to possess antimalarial behavior. It is important to highlight that while certain blood proteins might also get adsorbed on GOns surface,15,18,24,25 the proper functioning of RBCs should not be affected as their primary function in facilitating oxygen transportation would typically be affected only in the event of hemolysis.
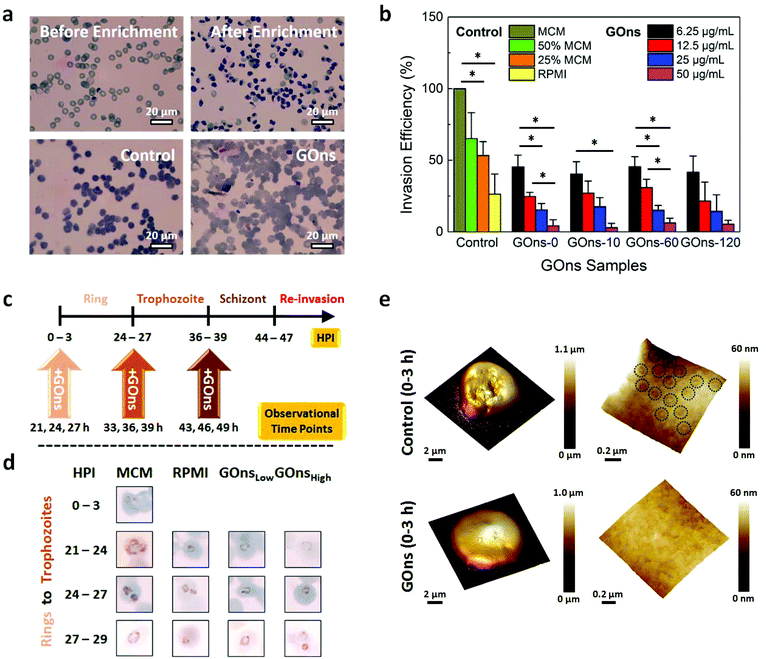
Following the elucidation of the possible mechanisms underlying the anticipated antimalarial behavior of GOns, we sought to evaluate their efficiency in inhibiting the invasion of P. falciparum parasites. Enriched infected erythrocytes of the laboratory adapted 3D7 strain were mixed with healthy blood and incubated in MCM in the presence of various GOns samples for 48 h. Smears for individual samples were made and stained with Giemsa before determining the parasitemia via counting approximately 1000 cells (Fig. 4a). The complete MCM and MCM diluted with different percentages of RPMI served as negative and positive controls, respectively. In light of the collated data, we observed that GOns exhibited a concentration-dependent antimalarial behavior by significantly inhibiting the parasite invasion of RBCs (Fig. 4b). Specifically, the invasion efficiency of the malaria parasites decreased progressively along with an increasing concentration of GOns from 6.25 μg mL−1 to 50 μg mL−1. Invasion efficiency is determined by normalizing the parasitemia to that of control, which is taken to be 100%. Intriguingly, even at a low concentration of 6.25 μg mL−1, the parasite invasion efficiency due to GOns was significantly lower than that of negative control and similar to that of 25% MCM. Further increase in the concentration of GOns to 50 μg mL−1 resulted in a greater decrease in the parasite invasion efficiency as compared to that of positive controls. It is interesting to note that all GOns exhibited similar parasite inhibition efficiency despite variations in albumax II adsorption on different GOns samples. As pointed out earlier, the antimalarial property of GOns might not be solely dependent on the depletion of nutrients through albumax II adsorption. GOns might also physically restrict the access of merozoites to RBCs. Therefore, this antimalarial characteristic of GOns was likely to be dependent on the intricate interplay between serum albumin adsorption and physical blocking of RBCs from merozoites.
Next, the effects of GOns on parasite maturation were examined. Interestingly, a recent study has demonstrated that P. falciparum parasites are capable of delaying their maturation in response to amino acid depletion in order to survive prolonged starvation.52 As such, we postulated the occurrence of a similar phenomenon if GOns were able to absorb sufficient amount of albumax II to starve the parasites. To verify this, we monitored the development of tightly synchronized parasites (3 h) at three specific transitional stages, i.e., from rings to trophozoites, from trophozoites to schizonts, and from schizonts to rings of the next life cycle, in the absence and presence of GOns. Here, GOns with two different effective concentrations of 6.25 μg mL−1 (GOnsLow) and 50 μg mL−1 (GOnsHigh) were added to the parasite cultures at 0 h, 24 h, and 36 h post-infection (HPI) when the synchronized parasite cultures were in the ring, trophozoite, and schizont stage, respectively (Fig. 4c). As before, MCM and MCM diluted with RPMI were correspondingly used as negative and positive controls. Smears of the cultures were taken at multiple HPIs, i.e., 24 ± 3 h, 36 ± 3 h, and 46 ± 3 h, to ensure that observations were not due to asynchrony of the cells. We observed that the progression of malaria parasites was delayed from rings to trophozoites in the presence of GOnsHigh and solely in RPMI (Fig. 4d). In fact, these parasites remained largely in the ring stage even when those cultured solely in MCM and in the presence of GOnsLow had progressed to trophozoites at 21 h, 24 h, and 27 h. As compared to GOnsLow, GOnsHigh exhibited a more pronounced effect in delaying the development of parasites from rings to trophozoites, plausibly due to the higher amount of albumax II adsorbed from the medium. Nevertheless, it is likely that the transition delay observed from rings to trophozoites was temporary considering that most of the infected cells were in the trophozoite stage when parasitemia was assessed after 48 h of culturing late stage infected cells in the previous section. Additionally, this stage transition delay was only evident when GOns were introduced while the parasites were still in the ring stage. The addition of GOns at 24 h and 36 h (i.e., when the parasites were in the trophozoite and schizont stage, respectively) did not hamper the maturation of these parasites to schizonts and rings, respectively (Fig. S6†).
To gain further insights into the GOns-induced delayed maturation of parasites from rings to trophozoites, we characterized the presentation of knobs on the surface of iRBCs in the absence and presence of GOns. One of the key features corresponding to the asexual growth of malaria parasites is the presentation of nanoscale protrusions or knobs on the iRBC surface due to the transport of knob-associated proteins from the parasites to erythrocytes.53,54 In fact, the quantity and size of knobs found on the iRBC surface have been demonstrated to be directly correlated to the stage of infection55 as knobs are typically manifested on iRBCs when the parasites have matured to trophozoite and beyond. As such, the denser and more concentrated the knobs exhibited on the iRBC surface, the more advanced the stage of parasite development.55 GOns were introduced into the malaria culture at 0–3 h and 24–27 h. The surface morphology of the cultured iRBC samples was then characterized at 39–42 h. For control cultures, densely distributed surface knobs were observed, as expected (Fig. 4e and Fig. S7†). However, when GOns were introduced at 0 h, the knobs on the surface of iRBCs could hardly be observed even at 39–42 h (Fig. 4e). These knob characterization results corroborated with our previous observation in which GOns delayed the maturation of malaria parasites from rings to trophozoites. Cultures with GOns added at 24–27 h also showed distinct knobs on the iRBC surface at 39–42 h (Fig. S7†). This indicates that GOns did not affect the trophozoite-stage iRBCs from expressing knobs on their surface.
3. Conclusions
In summary, we investigated the in vitro interactions between GOns and malaria parasites. The mechanisms leading to the possible antimalarial behavior of GOns, i.e., physical obstruction, merozoite adhesion, and nutrient depletion were first examined. The adhesion of merozoites to the physical barrier GOns could hinder the invasion of RBCs. Additionally, the high loading capacity of GOns for the constituents of MCM likely caused the depletion of nutrients necessary for the growth and invasion of P. falciparum parasites. We further demonstrated the reduction in parasite invasion efficiency by GOns. Experimental results showed that GOns displayed a concentration-dependent antimalarial behavior by significantly inhibiting the invasion of malaria parasites into RBCs. We also observed that GOns induced a stage transition delay which interrupted the maturation of malaria parasites from rings to trophozoites. Overall, we anticipate that this study will be beneficial for the further exploration of nanomaterials-based approaches in malaria research.4. Experimental section
GO nanosheet preparation and characterization
GOns in aqueous solution were first prepared based on Hummer's method. They were then subjected to ultrasonication treatment (SONICS, VCX-130) at 40% amplitude for 15 s ON and 5 s OFF cycles, for different durations of 10, 60, and 120 min to yield GOns with various lateral size distributions and mean lateral sizes. Atomic force microscope (AFM) (Bruker, Billerica, MA) operating under the tapping mode was used to characterize the surface morphological features of the GOns samples in liquid suspension dropped on freshly cleaved mica. Using ImageJ software (NIH, US), the lateral size distributions of GOns in aqueous solution were estimated from the obtained AFM images. Roughly 400 GOns were assessed to calculate their lateral size distributions and mean lateral sizes.Biomolecule loading capacity assay
Individual constituents of MCM – Albumax II (Gibco Invitrogen), hypoxanthine (Sigma Aldrich), and gentamicin reagent (Life Technologies) – were prepared in 1× PBS at a concentration of 2 mg mL−1. GOns in liquid suspension were prepared at different concentrations of 50, 100, and 200 μg mL−1. The MCM constituents (30 μL) were then mixed with GOns (30 μL) and the resultant mixtures were incubated at 37 °C for 48 h. After the incubation, the mixtures were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min and the supernatants were collected for spectrophotometric measurements. Using the UV-Vis spectrophotometer (NanoDrop 2000, Thermo Scientific), the adsorption isotherm of each MCM constituent was obtained. Three independent readings under room temperature were acquired for each absorption spectrum. The adsorption of each MCM constituent on GOns was subsequently determined from the difference in protein adsorption pre- and post-addition of GOns.
000g for 10 min and the supernatants were collected for spectrophotometric measurements. Using the UV-Vis spectrophotometer (NanoDrop 2000, Thermo Scientific), the adsorption isotherm of each MCM constituent was obtained. Three independent readings under room temperature were acquired for each absorption spectrum. The adsorption of each MCM constituent on GOns was subsequently determined from the difference in protein adsorption pre- and post-addition of GOns.
Sample preparation and AFM imaging
For imaging of iRBCs, the smears of suspension cultures (i.e., 2.5% hematocrit, 1% parasitemia with 10% of 62.5 μg mL−1 GOns of all sizes) were made and dried on a hotplate at 40 °C for 30 min. For imaging of the physical interactions between GOns, RBCs, and merozoites, magnetically enriched late stage iRBCs were incubated with RBCs at 2.5% hematocrit and 5% parasitemia as well as 10% of 62.5 μg mL−1 GOns of various sizes in the culture. A smear was made 12 h later when all iRBCs had burst and the merozoites had been released into the culture. Slides were stored in a dehumidified chamber before imaging. All samples were imaged with a Bruker Dimension FastScan AFM system operating in PeakForce Tapping mode. AFM images were acquired with a Bruker FastScan-B cantilever (spring constant 4 N m−1) at a maximum scan rate of 2 Hz and 512 × 512 data points per image. Raw AFM data were processed using Bruker Nanoscope Analysis 1.50 software.Merozoite adhesion assay, fluorescence staining, and imaging
100 μL liquid solutions of 50 μg mL−1 GOns with different lateral size distributions and mean lateral sizes were drop-casted on 18 mm glass coverslips and heated at 120 °C to produce solid GOns substrates. Approximately 200 μL of enriched infected erythrocyte suspension (0.5% by volume in MCM) was then seeded on each solid GOns substrate. After the iRBCs burst, these solid GOns substrates were washed gently in 1× PBS to remove any cellular components that did not stick onto the substrates. Eventually, after a five minute incubation with 10 mg mL−1 ethidium bromide, Zeiss confocal microscope was used to obtain the fluorescent images of the cellular contents adhered on the solid GOns substrates.Malaria invasion assay
Synchronized and enriched infected erythrocytes were first added to healthy blood (1% parasitemia) to form a mixture. In each well of the 96-well plate, 175 μL of malaria culture medium (MCM) was added together with 5 μL of infected blood mixture and 20 μL of the GOns samples (i.e., GOns-0, GOns-10, GOns-60, and GOns-120 at different concentrations of 62.5, 125, 250, and 500 μg mL−1). As a negative control, 5 μL of infected blood mixture was added to 175 μL of MCM and 20 μL of MilliQ water. Positive controls replaced MCM in the negative control with RPMI (i.e., 50%, 75%, and 100% RPMI). The filled 96-well plate was then incubated for 48 h. A smear was made for each well and stained with Giemsa after fixing with methanol. Each smear was observed under an optical microscope using a 100× oil objective. At least 1000 cells were counted for each smear to obtain the parasitemia. Three independent experiments were performed and the obtained results were averaged.Parasite stage development assay
Laboratory strain of Plasmodium falciparum, 3D7, was tightly synchronized with sorbitol prior to the experiment within a 3 h window. Three sets of cultures, consisting of 12 wells each, were seeded in a 96-well plate at 0–3 h post-infection with 0.5% parasitemia and 2.5% hematocrit. At 0 h, GOns in liquid suspension were introduced to the first set of cultures, i.e., four cultures with 10% of 62.5 μg mL−1 of GOns (i.e., GOns-0, GOns-10, GOns-60, and GOns-120) and four cultures with 10% of 500 μg mL−1 of GOns. For comparison, MCM in the remaining four cultures was replaced with varying amounts of RPMI such that the cultures contained 100% MCM, 50% MCM, 25% MCM, and 0% MCM. Smears were fixed and stained with Giemsa at 21, 24, and 27 h. Similar steps were performed on the second and third sets at 24 h (observed at different time points of 33, 36, and 39 h) and 36 h (observed at different time points of 43, 46, and 49 h), respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Kenry and Y. B. Lim contributed equally to this work. Kenry would like to acknowledge NUS Graduate School for Integrative Sciences and Engineering Scholarship. Y. B. Lim would like to acknowledge SMA Graduate Fellowship at SMART. K. P. Loh wishes to acknowledge funding support from NRF-investigatorship award NRF-NRF12015-01 “Graphene oxide a new class of catalytic, ionic and molecular sieving materials.” This research was supported by the National Research Foundation, Prime Minister's Office, Singapore under its medium-sized centre programme, Centre for Advanced 2D Materials and its Research Centre of Excellence, Mechanobiology Institute, as well as the MechanoBioEngineering Laboratory of the Department of Biomedical Engineering of the National University of Singapore.References
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- S. Eigler and A. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 7720–7738 CrossRef CAS PubMed.
- Kenry and C. T. Lim, ChemNanoMat, 2017, 3, 5–16 CrossRef CAS.
- Kenry, A. Geldert, X. Zhang, H. Zhang and C. T. Lim, ACS Sens., 2016, 1, 1315–1321 CrossRef CAS.
- A. Geldert, Kenry, X. Zhang, H. Zhang and C. T. Lim, Analyst, 2017, 142, 2570–2577 RSC.
- Kenry, A. Geldert, Z. Lai, Y. Huang, P. Yu, C. Tan, Z. Liu, H. Zhang and C. T. Lim, Small, 2017, 13, 1601925 CrossRef PubMed.
- S. A. Sydlik, S. Jhunjhunwala, M. J. Webber, D. G. Anderson and R. Langer, ACS Nano, 2015, 9, 3866–3874 CrossRef CAS PubMed.
- Y. Liu, D. Yu, C. Zeng, Z. Miao and L. Dai, Langmuir, 2010, 26, 6158–6160 CrossRef CAS PubMed.
- A. M. H. Ng, Kenry, C. Teck Lim, H. Y. Low and K. P. Loh, Biosens. Bioelectron., 2015, 65, 265–273 CrossRef CAS PubMed.
- Q. Liu, L. Wei, J. Wang, F. Peng, D. Luo, R. Cui, Y. Niu, X. Qin, Y. Liu, H. Sun, J. Yang and Y. Li, Nanoscale, 2012, 4, 7084–7089 RSC.
- J. Li, W. Zhang, T.-F. Chung, M. N. Slipchenko, Y. P. Chen, J.-X. Cheng and C. Yang, Sci. Rep., 2015, 5, 12394 CrossRef PubMed.
- W. C. Lee, C. H. Y. X. Lim, H. Shi, L. A. L. Tang, Y. Wang, C. T. Lim and K. P. Loh, ACS Nano, 2011, 5, 7334–7341 CrossRef CAS PubMed.
- W. C. Lee, C. H. Lim, Kenry, C. Su, K. P. Loh and C. T. Lim, Small, 2015, 11, 963–969 CrossRef CAS PubMed.
- T.-H. Kim, S. Shah, L. Yang, P. T. Yin, M. K. Hossain, B. Conley, J.-W. Choi and K.-B. Lee, ACS Nano, 2015, 9, 3780–3790 CrossRef CAS PubMed.
- Kenry, P. K. Chaudhuri, K. P. Loh and C. T. Lim, ACS Nano, 2016, 10, 3424–3434 CrossRef CAS PubMed.
- S. Liu, M. Hu, T. H. Zeng, R. Wu, R. Jiang, J. Wei, L. Wang, J. Kong and Y. Chen, Langmuir, 2012, 28, 12364–12372 CrossRef CAS PubMed.
- J. Zhao, B. Deng, M. Lv, J. Li, Y. Zhang, H. Jiang, C. Peng, J. Li, J. Shi, Q. Huang and C. Fan, Adv. Healthcare Mater., 2013, 2, 1259–1266 CrossRef CAS PubMed.
- Kenry, K. P. Loh and C. T. Lim, Small, 2015, 11, 5105–5117 CrossRef CAS PubMed.
- Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 130, 10876–10877 CrossRef CAS PubMed.
- X. Zhao, L. Yang, X. Li, X. Jia, L. Liu, J. Zeng, J. Guo and P. Liu, Bioconjugate Chem., 2015, 26, 128–136 CrossRef CAS PubMed.
- H. Yue, W. Wei, Z. Yue, B. Wang, N. Luo, Y. Gao, D. Ma, G. Ma and Z. Su, Biomaterials, 2012, 33, 4013–4021 CrossRef CAS PubMed.
- C. Chung, Y.-K. Kim, D. Shin, S.-R. Ryoo, B. H. Hong and D.-H. Min, Acc. Chem. Res., 2013, 46, 2211–2224 CrossRef CAS PubMed.
- V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Chem. Rev., 2016, 116, 5464–5519 CrossRef CAS PubMed.
- Kenry, K. P. Loh and C. T. Lim, Nanoscale, 2016, 8, 9425–9441 RSC.
- Kenry, K. P. Loh and C. T. Lim, RSC Adv., 2016, 6, 46558–46566 RSC.
- C. Fisher, A. E. Rider, Z. Jun Han, S. Kumar, I. Levchenko and K. Ostrikov, J. Nanomater., 2012, 2012, 315185 CrossRef.
- Y. Zhang, S. F. Ali, E. Dervishi, Y. Xu, Z. Li, D. Casciano and A. S. Biris, ACS Nano, 2010, 4, 3181–3186 CrossRef CAS PubMed.
- A. Sasidharan, L. S. Panchakarla, P. Chandran, D. Menon, S. Nair, C. N. R. Rao and M. Koyakutty, Nanoscale, 2011, 3, 2461–2464 RSC.
- K. Wang, J. Ruan, H. Song, J. Zhang, Y. Wo, S. Guo and D. Cui, Nanoscale Res. Lett., 2011, 6, 8 Search PubMed.
- M. Xu, J. Zhu, F. Wang, Y. Xiong, Y. Wu, Q. Wang, J. Weng, Z. Zhang, W. Chen and S. Liu, ACS Nano, 2016, 10, 3267–3281 CrossRef CAS PubMed.
- R. Kurapati, K. Kostarelos, M. Prato and A. Bianco, Adv. Mater., 2016, 28, 6052–6074 CrossRef CAS PubMed.
- P. D. Crompton, S. K. Pierce and L. H. Miller, J. Clin. Invest., 2010, 120, 4168–4178 CrossRef CAS PubMed.
- P. A. Buffet, I. Safeukui, G. Deplaine, V. Brousse, V. Prendki, M. Thellier, G. D. Turner and O. Mercereau-Puijalon, Blood, 2011, 117, 381–392 CrossRef CAS PubMed.
- B. Baragana, I. Hallyburton, M. C. S. Lee, N. R. Norcross, R. Grimaldi, T. D. Otto, W. R. Proto, A. M. Blagborough, S. Meister, G. Wirjanata, A. Ruecker, L. M. Upton, T. S. Abraham, M. J. Almeida, A. Pradhan, A. Porzelle, M. S. Martinez, J. M. Bolscher, A. Woodland, S. Norval, F. Zuccotto, J. Thomas, F. Simeons, L. Stojanovski, M. Osuna-Cabello, P. M. Brock, T. S. Churcher, K. A. Sala, S. E. Zakutansky, M. B. Jimenez-Diaz, L. M. Sanz, J. Riley, R. Basak, M. Campbell, V. M. Avery, R. W. Sauerwein, K. J. Dechering, R. Noviyanti, B. Campo, J. A. Frearson, I. Angulo-Barturen, S. Ferrer-Bazaga, F. J. Gamo, P. G. Wyatt, D. Leroy, P. Siegl, M. J. Delves, D. E. Kyle, S. Wittlin, J. Marfurt, R. N. Price, R. E. Sinden, E. A. Winzeler, S. A. Charman, L. Bebrevska, D. W. Gray, S. Campbell, A. H. Fairlamb, P. A. Willis, J. C. Rayner, D. A. Fidock, K. D. Read and I. H. Gilbert, Nature, 2015, 522, 315–320 CrossRef CAS PubMed.
- C. Dogovski, S. C. Xie, G. Burgio, J. Bridgford, S. Mok, J. M. McCaw, K. Chotivanich, S. Kenny, N. Gnädig, J. Straimer, Z. Bozdech, D. A. Fidock, J. A. Simpson, A. M. Dondorp, S. Foote, N. Klonis and L. Tilley, PLos Biol., 2015, 13, e1002132 Search PubMed.
- A. Harikishore, M. Niang, S. Rajan, P. R. Preiser and H. S. Yoon, Sci. Rep., 2013, 3, 2501 CrossRef PubMed.
- A. F. Cowman, D. Berry and J. Baum, J. Cell Biol., 2012, 198, 961–971 CrossRef CAS PubMed.
- J.-H. Liu, S.-T. Yang, H. Wang, Y. Chang, A. Cao and Y. Liu, Nanomedicine, 2012, 7, 1801–1812 CrossRef CAS PubMed.
- F. Perreault, A. F. de Faria, S. Nejati and M. Elimelech, ACS Nano, 2015, 9, 7226–7236 CrossRef CAS PubMed.
- F. Zhang, F. Liu, C. Wang, X. Xin, J. Liu, S. Guo and J. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 2104–2110 CAS.
- C. G. Salzmann, V. Nicolosi and M. L. H. Green, J. Mater. Chem., 2010, 20, 314–319 RSC.
- L. H. Bannister, J. M. Hopkins, A. R. Dluzewski, G. Margos, I. T. Williams, M. J. Blackman, C. H. Kocken, A. W. Thomas and G. H. Mitchell, J. Cell Sci., 2003, 116, 3825–3834 CrossRef CAS PubMed.
- F. L. Schuster, Clin. Microbiol. Rev., 2002, 15, 355–364 CrossRef PubMed.
- K. Singh, A. Agarwal, S. I. Khan, L. A. Walker and B. L. Tekwani, J. Biomol. Screening, 2007, 12, 1109–1114 CrossRef CAS PubMed.
- S. A. Desai, Sci. World J., 2013, 2013, 363505 Search PubMed.
- P. T. Hammond, Mater. Today, 2012, 15, 196–206 CrossRef CAS.
- Z. Ding, H. Ma and Y. Chen, RSC Adv., 2014, 4, 55290–55295 RSC.
- R. Feng, Y. Yu, C. Shen, Y. Jiao and C. Zhou, J. Biomed. Mater. Res., Part A, 2015, 103, 2006–2014 CrossRef CAS PubMed.
- M. Šimšíková, Arch. Biochem. Biophys., 2016, 593, 69–79 CrossRef PubMed.
- A. El Tahir, P. Malhotra and V. Chauhan, Malar. J., 2003, 2, 11 CrossRef PubMed.
- J. Liu, E. S. Istvan, I. Y. Gluzman, J. Gross and D. E. Goldberg, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8840–8845 CrossRef CAS PubMed.
- S. E. Babbitt, L. Altenhofen, S. A. Cobbold, E. S. Istvan, C. Fennell, C. Doerig, M. Llinás and D. E. Goldberg, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E3278–E3287 CrossRef CAS PubMed.
- M. Rug, S. W. Prescott, K. M. Fernandez, B. M. Cooke and A. F. Cowman, Blood, 2006, 108, 370–378 CrossRef CAS PubMed.
- Y. Zhang, C. Huang, S. Kim, M. Golkaram, M. W. A. Dixon, L. Tilley, J. Li, S. Zhang and S. Suresh, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 6068–6073 CrossRef CAS PubMed.
- A. Li, A. H. Mansoor, K. S. W. Tan and C. T. Lim, J. Microbiol. Methods, 2006, 66, 434–439 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nr06007f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |