MoS2 nanosheets encapsulated in sodium alginate microcapsules as microwave embolization agents for large orthotopic transplantation tumor therapy†
Abstract
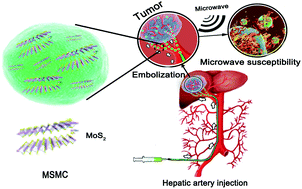
In recent years, it is prevalent to treat various kinds of the tumors through microwave ablation method. However, it is still very difficult to ablate large tumors by the traditional microwave ablation therapy. In this work, an effective microwave embolization agent designed by encapsulating molybdenum sulfide nanosheets in the sodium alginate microcapsules, denoted as MSMCs, was prepared for the effective therapy of large tumor. The toxicity evaluation showed that MSMC had a good biocompatibility in vitro. The in vitro and in vivo experiments demonstrated that the MSMC was an excellent embolic and microwave susceptible agent that could be used for dual-enhanced microwave ablation therapy. As such, the MSMC showed excellent tumor therapeutic effect with 5 times larger ablation zone observed by magnetic resonance (MR) imaging than the microwave alone after 3 days treating. Besides, the tumor is nearly completely ablated and can not be recurrent due to the persistent hyperthermia. Moreover, MSMCs have a good biocompatibility and can be degraded and cleared from the body. It is believed that the MSMC is demonstrated to be a promising multifunctional theranostic agent used for treating the larger tumor via the synergistic therapy of enhanced microwave ablation and transcatheter arterial embolization (TAE).


 Please wait while we load your content...
Please wait while we load your content...