A general method for ultrathin 1D oxide nanomaterials†
Abstract
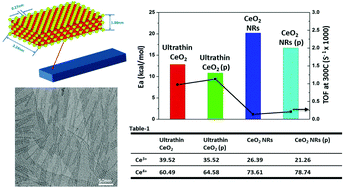
By utilizing the interaction between inorganic species and organic surfactants, the ordered layered mesostructures were generated for the synthesis of the one-dimensional oxide nanomaterials. The oxide nanomaterial products which evolved from the above layered structures were demonstrated as ultrathin (less than 2 nm) one-dimensional structures with superior catalytic performance. The synthetic method based on layered structures can be extended to prepare other one-dimensional oxide nanomaterials with the same ultrathin structures.


 Please wait while we load your content...
Please wait while we load your content...