Wafer-scale production of vertical SnS multilayers for high-performing photoelectric devices†
Abstract
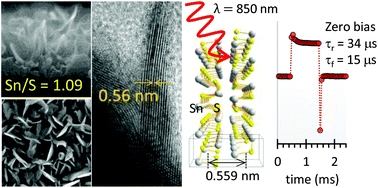
This study achieved wafer-scale, high quality tin monosulfide (SnS) layers. By using a solid-state reaction, the vertically aligned SnS layers spontaneously grew with sulphur reduction from the sputtered SnS2 particles without any post processes. The quality of the SnS vertical layers was observed by high resolution transmission electron microscopy, which confirmed an interlayer space of 0.56 nm for a perfect match to the theoretical value. The phase purity of SnS was confirmed by Raman spectroscopy. The intrinsic energy band gap value (1.6 eV) of SnS is attractive for photoelectric devices. To form a heterojunction, the vertical SnS layers were grown on a n-type Si substrate. Due to the nanoscale size and vertical standing features of the SnS layers, a significantly low reflection (<5%) was realized for the SnS/n-Si heterojunction device. As a photovoltaic cell, the device provides a higher open circuit voltage (>300 mV). For photodetection, the response speed is faster than 15 μs for near infrared wavelength photons, which is a 1000 times improvement over the horizontally shaped device. The vertically standing SnS layers show high photoreactive performance, which confirms that the functional design of 2D materials is an effective route to achieve enhanced photoelectric devices, such as photodetectors and solar cells.


 Please wait while we load your content...
Please wait while we load your content...