Injectable thermosensitive magnetic nanoemulsion hydrogel for multimodal-imaging-guided accurate thermoablative cancer therapy†
Abstract
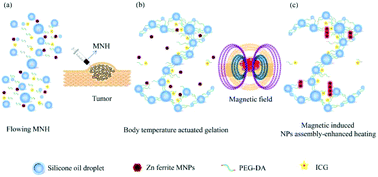
Ferrofluid-based magnetic hyperthermia of cancers has gained significant attention in recent years due to its excellent efficacy, few deleterious side effects and unlimited tissue penetration capacity. However, the high tumor osmotic pressure causes injection leakage and thus position imprecision because of the fluidity of the ferrofluid and the absence of multimodal imaging guidance, which create tremendous challenges for clinical application. Here, a body temperature-induced gelation strategy is constructed for accurate localized magnetic tumor regression based on the unique behaviors of a magnetic nanoemulsion hydrogel (MNH) within tumors. The rapid intra-tumor gelation can securely restrict the MNH in tumor tissue without diffusion and leakage. The magnetically induced nanoparticle assembly-enhanced heating in the hydrogel and the heat accumulation caused by crosslinking among the nanoemulsion droplets further increased the heating efficiency. Meanwhile, US/MR/NIR multimodal imaging can guide the whole therapeutic process, achieving excellent magnetic hyperthermia therapeutic efficiency. This work highlights the great promise for improving the magnetic hyperthermia efficiency and the precision of the injection site for localized tumor therapy.


 Please wait while we load your content...
Please wait while we load your content...