Open Access Article
Open Access ArticleAn organometallic route to chiroptically active ZnO nanocrystals†
Elżbieta
Chwojnowska
ab,
Małgorzata
Wolska-Pietkiewicz
b,
Justyna
Grzonka
ac and
Janusz
Lewiński
 *ab
*ab
aInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: lewin@ch.pw.edu.pl
bFaculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
cFaculty of Materials Science and Engineering, Warsaw University of Technology, Wołoska 141, 02-507 Warsaw, Poland
First published on 19th July 2017
Abstract
The unique optical properties of zinc oxide nanocrystals (ZnO NCs) are strongly dependent on both the properties and the composition of the inorganic core–organic ligand interface. Developing a novel organometallic self-supporting approach, we report on the synthesis and characterization of ZnO nanocrystals coated by chiral monoanionic aminoalcoholate ligands. The resulting ZnO NCs are both chiroptically active and possess size dependent optical properties. The size and in consequence the emission color of the ZnO NCs could be simply adjusted by the characteristic of the aminoalcohol used.
Chirality is one of the most important factors of molecular recognition in nature and plays a tremendous role in various areas of chemistry. Although chiroptical activity as a common natural phenomenon is satisfactorily well understood at the molecular level, nanoscale chirality is a very new area of research.1–4 The concept of ‘nanochirality’ offers an intriguing route to engineer advanced nanomaterials with novel properties, and develops a broad range of promising applications in photonics, biomedicine, optics, catalysis, and sensing. In this context, the development of chiroptically active colloidal semiconductor nanocrystals and understanding of their optical activity have been an emerging issue of nanoresearch over the past decade. To our knowledge, there are only a handful of examples for the synthesis and characterization of chiroptically active semiconductor NCs or quantum dots (QDs), which essentially concern cadmium-based nanomaterials (CdX NCs, where X = S, Se or Te). Generally, these NCs were coated by various optically active biomolecules (e.g. penicillamine,5–10 cysteine and its derivatives11–13 or glutathione14,15), and were prepared mostly via standard procedures using chiral stabilizing ligands or by using post-synthetic ligand exchange methods.16–18 Remarkably, there is a lack of examples of optically active colloidal semiconductor nanocrystals or quantum dots of ZnO to date.19
In the course of our investigations on the development of efficient strategies for the preparation of various ZnO nanocrystalline materials,20–25 herein we report on an effective organometallic strategy for chiroptically active colloidal ZnO nanocrystals using easy-to-prepare ethylzinc complexes incorporating homochiral aminoalcoholate ligands as organometallic precursors (Scheme 1). We anticipated that the utility of organozinc derivatives of chiral aminoalcohols can give a chance to introduce ligands with a strongly anchoring O-alkoxide group situated next to an asymmetric carbon atom onto the ZnO NC surface, which can potentially pave the way to induce chirality into the nanocrystal–ligand interface. These predictions were verified in the following experiments.
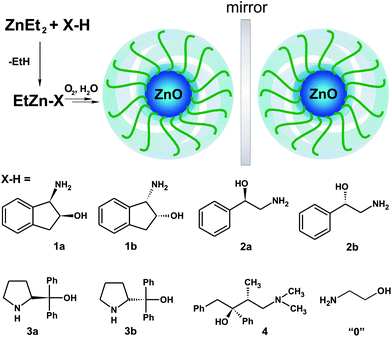 | ||
| Scheme 1 Schematic representation of a novel organometallic approach for the preparation of aminoalcoholate-coated chiral ZnO NCs. | ||
Even though alkylzinc alkoxides have been used as predesigned single-source precursors of ZnO-based nanostructures,26–29 and amines are widely used as neutral type stabilizing ligands for ZnO NCs,30–34 we note that the application of organozinc aminoalcoholates as organozinc precursors of ZnO nanomaterials still remains an essentially unexplored area.35 To gain more in-depth insight into the factors determining the properties of chiral aminoalcoholate coated ZnO NCs, a series of structurally diverse chiral α- and β-aminoalcohols were selected as proligands, i.e. (1R,2S)- and (1S,2R)-cis-1-amino-2-indanol (denoted as 1a and 1b, respectively), (R)- and (S)-2-amino-1-phenylethanol (2a and 2b), (R)- and (S)-α,α-diphenyl-2-pyrrolidinemethanol (3a and 3b) and (2S,3R)-4-dimethylamino-3-methyl-1,2-diphenyl-2-butanol (4)36 (Scheme 1). Our synthetic protocol for the preparation of ZnO NCs with the surface passivated by monoanionic aminoalcoholate ligands involves a one-pot two-step synthetic procedure shown in Scheme 1. In the first step, [EtZn(O,N)]-type complexes were synthesized in situ by the equimolar reaction of a commercially available Et2Zn and the selected aminoalcohol.37 Then, a THF solution of the corresponding EtZn(O,N) precursor was exposed to air at ambient temperature to initiate transformations leading to (O,N)-ligand-coated ZnO NCs (for experimental details see the ESI†). The full transformations of the organometallic precursors were achieved within ca. 4–5 days. Then the resulting ZnO NCs were separated from the parent THF solution and purified. The structural and physical properties of the resulting ZnO NCs were studied by using high-resolution transmission electron microscopy (HR-TEM), powder X-ray diffraction (PXRD), dynamic light scattering (DLS), UV-vis absorption, circular dichroism (CD) and photoluminescence (PL) spectroscopy.
Initially, achiral 2-aminoethanol (“0”) was chosen to probe an organometallic approach to aminoalcoholate-coated ZnO nanocrystals. Using a one-pot two-step organometallic approach we successfully synthesized ZnO NCs coated by the 2-aminoethanolate ligands (denoted as ZnO-“0”, Scheme 1), which formed a suspension in THF and a colloidal solution in DMSO. The HR-TEM images show that the exposure of [EtZn(O,N)]-type precursors toward air affords well-dispersed and quasi-spherically shaped nanoparticles with a narrow size distribution and a mean core diameter of 3.2 ± 0.4 nm (Fig. S1†). The PXRD pattern for ZnO-“0” is consistent with a model pattern of a hexagonal wurtzite-type ZnO phase (Fig. S3†). The ZnO-“0” NCs revealed a broad absorption that dropped at λ = 350 nm in the UV region and a PL spectrum emission peak centered at λ = 512 nm (FWHM = 129 nm) (Fig. S5†).
Subsequently, a series of optically active ZnO NCs coated by chiral aminoalcoholate ligands were obtained using the same synthetic procedure (Scheme 1). The resulting ZnO NCs stabilized by cis-1-amino-2-indanolate (ZnO-1 NCs) and 2-amino-1-phenylethanolate (ZnO-2 NCs) form readily colloidal solutions in THF and their hydrodynamic diameters were found to be 2.7 nm and 4 nm, respectively (Fig. S6 and S7†). In contrast, ZnO NCs stabilized by α,α-diphenyl-2-pyrrolidinemethanolate (ZnO-3 NCs) and (2S,3R)-4-dimethylamino-3-methyl-1,2-diphenyl-2-butanolate (ZnO-4 NCs) have a strong tendency for aggregation in THF and form colloidal solutions in DMSO. The average hydrodynamic diameter of ZnO-3 NCs and ZnO-4 NCs dispersed in DMSO was 12 nm and 17 nm, respectively (Fig. S8 and S9†). The HR-TEM micrographs (Fig. 1 and Fig. S10–S22†) show quasi-spherically shaped nanoparticles with average core diameters of 1.7 ± 0.2 nm, 2.9 ± 0.4 nm, 7.5 ± 1.4 nm and 8.0 ± 1.6 nm for ZnO-1, ZnO-2, ZnO-3 and ZnO-4 NCs, respectively. The phase purity and the degree of crystallinity of ZnO-1–ZnO-4 NCs were confirmed by PXRD measurements (Fig. S24†) and the observed diffraction patterns corresponded well with that of the standard wurtzite-type ZnO phase. The data clearly indicate that inorganic core diameters of the resulting ZnO NCs differ significantly and strongly depend on the characteristic of the aminoalcoholate ligand used. For example, ZnO-1 NCs coated by α-aminoalcoholate ligands are significantly smaller in size than the NCs supported by β-aminoalcoholates (ZnO-4 NCs). It is also likely that both conformational and steric factors have influence on the size of aminoalcoholate ligand-coated ZnO NCs (e.g. the smallest nanoparticles with 1.7 nm core diameter were obtained from the transformation of the [EtZn(O,N)]-type precursor incorporating the conformationally constrained aminoindolate ligands).
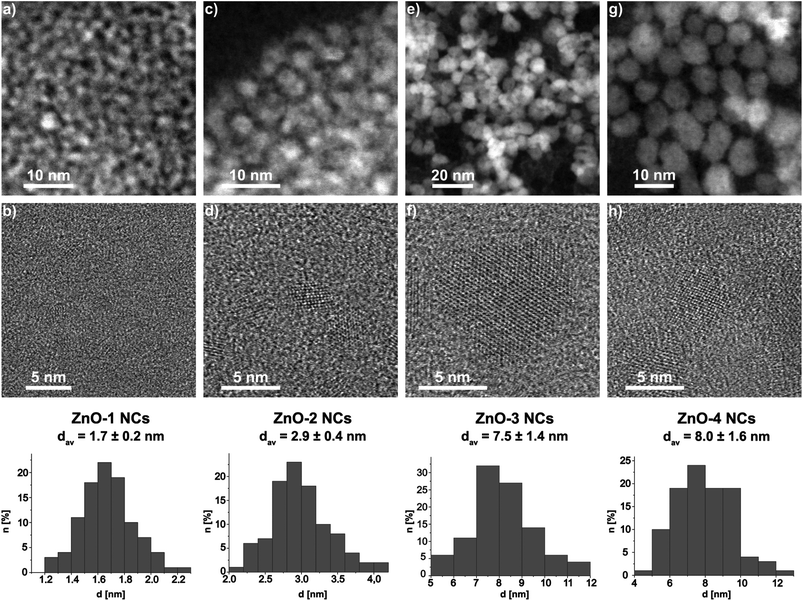 | ||
| Fig. 1 HAADF STEM (top) and HR-TEM (bottom) micrographs of (a, b) ZnO-1, (c, d) ZnO-2, (e, f) ZnO-3, (g, h) ZnO-4 and their size distributions. | ||
We were particularly interested in the optical activity of the obtained ZnO NCs. In order to acquire knowledge about the chiroptical properties UV-visible absorption and circular dichroism (CD) spectra were measured for the resulting aminoalcoholate-coated ZnO NCs and the corresponding proligands (Fig. 2). The ZnO NCs revealed a characteristic broad absorption that dropped at 280 nm, 324 nm, 350 nm and 345 nm in the ultraviolet region, for ZnO-1, ZnO-2, ZnO-3 and ZnO-4, respectively. All of the studied ZnO NCs were found to be optically active with the electronic CD spectra exhibiting symmetrical mirror-image profiles (Fig. 2; for ZnO-4, the CD spectrum of the NCs obtained from only one commercially available enantiomer is presented). The data univocally demonstrate that aminoalcoholate ligands coating the NC surfaces preserve their own chirality. Interestingly, a comparison of the CD spectra of proligands 1a and 1b, and the corresponding ZnO-1a NCs and ZnO-1b NCs clearly showed the appearance of additional CD bands in the region from 260 nm to 360 nm. We thus believe that it is reasonable to hypothesize that these additional features could be attributed to a structural distortion of the NC surface atoms due to their interaction with the chiral capping ligands. It is worth noting that for ZnO-2 NCs weak and broad CD features between 290 and 340 nm can also be seen (Fig. 2b) but they are less conclusive due to their weakness. However, the diffuse shape in the CD signal can be attributed to a spectrum of the chiral units on the NC cores, especially in the range of 300–340 nm, where NCs have strong absorption. We have also noted that ZnO-3 and ZnO-4 essentially exhibit only CD features related to the aminoalcoholate ligands.
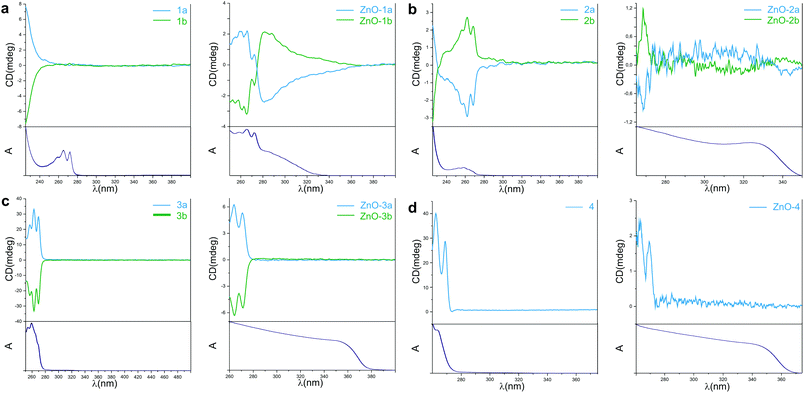 | ||
| Fig. 2 CD and normalized absorption spectra of proligands and ZnO NCs: (a) 1a, 1b, ZnO-1a, ZnO-1b, (b) 2a, 2b, ZnO-2a, ZnO-2b, (c) 3a, 3b, ZnO-3a, ZnO-3b and (d) 4, ZnO-4. | ||
As we noted above, the resulting ZnO NCs possess the ZnO wurtzite phase, which is characterized by the tetrahedral geometry of both zinc and oxide centers present in the crystal structure. For this reason, to understand the origin of optical activity in NCs the classical concept of chirality based on the tetrahedral geometry of sp3 hybridized atoms could be applied (this approach was previously proposed by Kotov et al. to explain the optical activity of CdTe NCs11). The deprotonated aminoalcoholate has two potential binding sites, i.e. the strongly anchoring anionic O-alkoxide group and the neutral pendant N-donor terminus, which can potentially chelate the neighbouring surface zinc atoms and form 5- or 6-membered chelate rings.38 The applied aminoalcoholates differ in flexibility of their backbone. For example, 1 has the relatively rigid backbone that allows easy bonding to surface atoms by both alkoxide and amine groups. This is not the case for 3 and 4 for which the character of the backbone hinder the formation of chelate entities due to the steric encumbrance or long and flexible backbones, respectively. Generally, aminoalcoholate ligand molecules bound with the ZnO NC surface preserve their own chirality. However, the ligand character may be responsible for differences observed in CD for ZnO-1 and ZnO-2 NCs where possibly additional chirality could be induced on the ZnO NC surface. Notably, the origin of the optical activity in semiconductor nanocrystals is a very complex issue and several different possible mechanisms of introducing optical activity in these nanostructures can be considered.39 Thus, there are different possible effects, which can be responsible for the observed chiroptical activity of the investigated ZnO NCs. At this stage of studies the origin of the observed optical activity is yet far from an explicit explanation and undoubtedly, further advanced research is demanded.
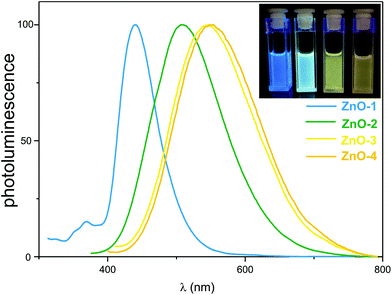
The photoluminescence spectra of ZnO-1, ZnO-2, ZnO-3 and ZnO-4 NCs showed broad PL emission peaks centered at 440 nm (FWHM = 64 nm), 508 nm (FWHM = 123 nm), 546 nm (FWHM = 140 nm) and 550 nm (FWHM = 141 nm), respectively (Fig. 3).40 Remarkably, the order of the peak positions is in good agreement with the increase in the size of ZnO NCs. Thus, these results show that the developed organometallic approach affords ZnO NCs with tunable luminescence colours just for one ligand family, i.e. the emission colour can be tuned from blue (ZnO-1) to green (ZnO-2) and yellow (ZnO-3 and ZnO-4) by rational adjustment of the stabilizing ligand. We note that tuning of the luminescence colour exhibited by ZnO NCs remains a very challenging issue. So far, only a handful of examples have been reported in which the emission colour of ZnO NCs was tuned by time-,41 concentration-42 or pH-dependent43 modifications of precipitation methods.
Conclusions
In conclusion, we have successfully developed an efficient one-pot two-step synthetic procedure for the preparation of novel chiroptically active ZnO NCs coated by strongly anchored aminoalcoholate ligand shells, using easy to prepare ethylzinc aminoalcoholates. The observed chiroptical activity of the resulting NCs differs significantly and strongly depends on the characteristic of the aminoalcoholate ligand used. Moreover, the presented data clearly indicate that the structure of the aminoalcoholate capping ligand has also a pronounced effect on both the inorganic core size as well as the tunable emission colour. Combined with a large pool of available chiral aminoalcohols and other protic organic proligands, our work opens a robust approach to the rational design of unique optically active ZnO-based semiconducting nanomaterials. Further studies on the fabrication of chiroptically active ZnO-based nanomaterials and the origin of their optical activity are in progress.Acknowledgements
The authors acknowledge the Foundation for Polish Science Team Program co-financed by the EU “European Regional Development Fund” TEAM/2011-7/8 and the National Science Centre (OPUS 2014/13/B/ST5/04420) for financial support.Notes and references
- Chirality at the Nanoscale: Nanoparticles, Surfaces, Materials and More, ed. E. Bonifazi, Wiley-VCH, Weinheim, 2009 Search PubMed.
- F. P. Milton, J. Govan, M. V. Mukhina and Y. K. Gun'ko, Nanoscale Horiz., 2016, 1, 14–26 RSC.
- J. Kumar, K. G. Thomas and L. M. Liz-Marzán, Chem. Commun., 2016, 52, 12555–12569 RSC.
- A. Ben-Moshe, S. G. Wolf, M. B. Sadan, L. Houben, Z. Fan, A. O. Govorov and G. Markovich, Nat. Commun., 2014, 5, 4302 CAS.
- M. P. Moloney, Y. K. Gun'ko and J. M. Kelly, Chem. Commun., 2007, 3900–3902 RSC.
- S. D. Elliott, M. P. Moloney and Y. K. Gun'ko, Nano Lett., 2008, 8, 2452–2457 CrossRef CAS PubMed.
- J. E. Govan, E. Jan, A. Querejeta, N. A. Kotov and Y. K. Gun'ko, Chem. Commun., 2010, 46, 6072–6074 RSC.
- A. B. Moshe, D. Szwarcman and G. Markovich, ACS Nano, 2011, 5, 9034–9043 CrossRef PubMed.
- S. A. Gallagher, M. P. Moloney, M. Wojdyla, S. J. Quinn, J. M. Kelly and Y. K. Gun'ko, J. Mater. Chem., 2010, 20, 8350–8355 RSC.
- M. Wojdyla, S. A. Gallagher, M. P. Moloney, Y. K. Gun'ko, J. M. Kelly, L. M. Magno, S. J. Quinn, I. P. Clark, G. M. Greetham and M. Towrie, J. Phys. Chem. C, 2012, 116, 16226–16232 CAS.
- Y. Zhou, M. Yang, K. Sun, Z. Tang and N. A. Kotov, J. Am. Chem. Soc., 2010, 132, 6006–6013 CrossRef CAS PubMed.
- J. Li, T. Yang, W. H. Chan, M. M. F. Choi and D. J. Zhao, J. Phys. Chem. C, 2013, 117, 19175–19181 CAS.
- T. Nakashima, Y. Kobayashi and T. Kawai, J. Am. Chem. Soc., 2009, 131, 10342–10343 CrossRef CAS PubMed.
- Y. Zhou, Z. Zhu, W. Huang, W. Liu, S. Wu, X. Liu, Y. Gao, W. Zhang and Z. Tang, Angew. Chem., Int. Ed., 2011, 50, 11456–11459 CrossRef CAS PubMed.
- Y. Li, Y. Zhou, H. Y. Wang, S. Perrett, Y. Zhao, Z. Tang and G. Nie, Angew. Chem., Int. Ed., 2011, 50, 5860–5864 CrossRef CAS PubMed.
- U. Tohgha, K. Varga and M. Balaz, Chem. Commun., 2013, 49, 1844–1846 RSC.
- C. Carrillo-Carrion, S. Cárdenas, B. M. Simonet and M. Valcárcel, Anal. Chem., 2009, 81, 4730–4733 CrossRef CAS PubMed.
- T. Delgado-Pérez, L. M. Bouchet, M. de la Guardia, R. E. Galian and J. Pérez-Prieto, Chem. – Eur. J., 2013, 19, 11068–11076 CrossRef PubMed.
- For the only one example of optically active nanostructured ZnO films, see: Y. Duan, L. Han, J. Zhang, S. Asahina, Z. Huang, L. Shi, B. Wang, Y. Cao, Y. Yao, L. Ma, C. Wang, R. K. Dukor, L. Sun, C. Jiang, Z. Tang, L. A. Nafie and S. Che, Angew. Chem., Int. Ed., 2015, 54, 15170–15175 CrossRef CAS PubMed.
- A. M. Cieślak, M. V. Pavliuk, L. D'Amario, M. Abdellah, K. Sokołowski, U. Rybinska, D. L. A. Fernandes, M. K. Leszczyński, F. Mamedov, A. M. El-Zhory, J. Föhlinger, A. Budinská, M. Wolska-Pietkiewicz, L. Hammarström, J. Lewiński and J. Sá, Nano Energy, 2016, 30, 187–192 CrossRef.
- J. Paczesny, M. Wolska-Pietkiewicz, I. Binkiewicz, M. Wadowska, Z. Wróbel, K. Matuła, W. Nogala, J. Lewiński and R. Hołyst, ACS Appl. Mater. Interfaces, 2016, 8, 13532–13541 CAS.
- A. Grala, M. Wolska-Pietkiewicz, W. Danowski, Z. Wrobel, J. Grzonka and J. Lewinski, Chem. Commun., 2016, 52, 7340–7343 RSC.
- P. Krupiński, A. Kornowicz, K. Sokołowski, A. M. Cieślak and J. Lewiński, Chem. – Eur. J., 2016, 22, 7817–7823 CrossRef PubMed.
- J. Paczesny, M. Wolska-Pietkiewicz, I. Binkiewicz, Z. Wróbel, M. Wadowska, K. Matuła, I. Dzięcielewski, D. Pociecha, J. Smalc-Koziorowska, J. Lewiński and R. Hołyst, Chem. – Eur. J., 2015, 21, 16941–16947 CrossRef CAS PubMed.
- K. Sokołowski, I. Justyniak, W. Bury, J. Grzonka, Z. Kaszkur, Ł. Mąkolski, M. Dutkiewicz, A. Lewalska, D. Kubicki, K. Wójcik, K. Kurzydłowski and J. Lewiński, Chem. – Eur. J., 2015, 21, 5488–5495 CrossRef PubMed.
- T. Omata, K. Takahashi, S. Hashimoto, Y. Maeda, K. Nose, S. Otsuka, Y. Matsuo and K. Kanaori, J. Colloid Interface Sci., 2011, 355, 274–281 CrossRef CAS PubMed.
- C. G. Kim, K. Sung, T. Chung, D. Y. Jung and Y. Kim, Chem. Commun., 2003, 16, 2068–2069 RSC.
- C. L. Carnes and K. J. Klabunde, Langmuir, 2000, 16, 3764–3772 CrossRef CAS.
- C. Lizandara-Pueyo, M. W. E. van den Berg, A. De Toni, T. Goes and S. Polarz, J. Am. Chem. Soc., 2008, 130, 16601–16610 CrossRef CAS PubMed.
- C. R. Bhattacharjee, D. D. Purkayastha and N. Das, Mater. Lett., 2012, 86, 108–111 CrossRef CAS.
- M. Kahn, A. Glaria, C. Pages, M. Monge, L. S. Macary, A. Maisonnat and B. Chaudret, J. Mater. Chem., 2009, 19, 4044–4060 RSC.
- Y. Coppel, G. Spataro, C. Pages, B. Chaudret, A. Maisonnat and M. L. Kahn, Chem. – Eur. J., 2012, 18, 5384–5393 CrossRef CAS PubMed.
- M. Shim and P. Guyot-Sionnest, J. Am. Chem. Soc., 2001, 123, 11651–11654 CrossRef CAS PubMed.
- M. L. Kahn, T. Cardinal, B. Bousquet, M. Monge, V. Jubera and B. Chaudret, ChemPhysChem, 2006, 7, 2392–2397 CrossRef CAS PubMed.
- There is one report on the preparation of ZnO thin films by low pressure chemical vapour deposition of methylzinc incorporating an aminoalcoholate ligand; A. L. Johnson, N. Hollingsworth, G. Kociok-Kohn and K. C. Molloy, Inorg. Chem., 2008, 47, 12040–12048 CrossRef CAS PubMed.
- There is only one commercially available enantiomer of 4-dimethylamino-3-methyl-1,2-diphenyl-2-butanol.
- It is well established that an equimolar reaction of dialkylzincs with aminoalcohols leads to the formation of [RZn(O,N)]-type alkylzinc compounds, see for example ref. 10.
- Considering that the chiral structure induced by chiral ligands is on the NC surface, the aminoalcoholate ligands can bound via O and N to one surface-Zn center, and introduce chirality via additional binding of (μ2-O) with a neighbouring surface-Zn.
- A. Ben-Moshe, A. Teitelboim, D. Orn and G. Markovich, Nano Lett., 2016, 16, 7467–7473 CrossRef CAS PubMed.
- PL spectra of ZnO-1a and ZnO-1b, ZnO-2a and ZnO-2b, ZnO-3a and ZnO-3b are essentially identical and in Fig. 3 only one representative spectrum is shown.
- L. Zhang, L. Yin, C. Wang, N. lun, Y. Qi and D. Xiang, J. Phys. Chem. C, 2010, 114, 9651–9658 CAS.
- K. F. Lin, H. M. Cheng, H. C. Hsu, L. J. Lin and W. F. Hsieh, Chem. Phys. Lett., 2005, 409, 208–211 CrossRef CAS.
- X. Tang, E. S. G. Choo, L. Li, J. Ding and J. Xue, Chem. Mater., 2010, 22, 3383–3388 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional HR TEM, PXRD, DLS, UV-Vis, CD, and PL data. See DOI: 10.1039/c7nr02843a |
| This journal is © The Royal Society of Chemistry 2017 |