Photosensitizer (PS)/polyhedral oligomeric silsesquioxane (POSS)-crosslinked nanohybrids for enhanced imaging-guided photodynamic cancer therapy†
Abstract
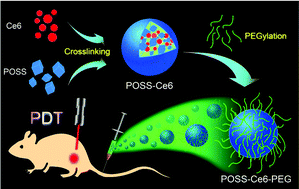
Photodynamic therapy (PDT) has drawn extensive attention as a promising cancer treatment modality. However, most PDT nanoagents suffer from insufficient drug loading capacity, a severe self-quenching effect, premature release of drugs and/or potential toxicity. Herein, we rationally designed an inorganic–organic nanohybrid with high drug loading capacity and superior chemical stability for enhanced PDT. Polyhedral oligomeric silsesquioxane (POSS), an amine-containing cage-shaped building block, was crosslinked with chlorin e6 (Ce6), a carboxyl-containing photosensitizer, via the amine–carboxyl reaction. Polyethylene glycol (PEG) polymers were further modified on the surface of the nanoparticle to improve the aqueous dispersibility and prolong the circulation time of the final nanoconstruct (POSS-Ce6-PEG). The as-prepared POSS-Ce6-PEG has a considerably high loading rate of Ce6 (19.8 wt%) with desirable fluorescence emission and singlet oxygen generation. Besides, in vitro experiments revealed that the nanoagent exhibited enhanced cellular uptake and a preferred intracellular accumulation within mitochondria and the endoplasmic reticulum, resulting in high anticancer efficiency under light irradiation. Furthermore, in vivo imaging-guided PDT was also successfully achieved, showing the effective tumor targeting and ablation ability of POSS-Ce6-PEG. More importantly, the nanoagent possesses negligible dark cytotoxicity and systemic side effects. Therefore, POSS-Ce6-PEG as an eligible PDT theranostic agent holds great potential in clinical applications.


 Please wait while we load your content...
Please wait while we load your content...