Ultralight, highly thermally insulating and fire resistant aerogel by encapsulating cellulose nanofibers with two-dimensional MoS2†
Abstract
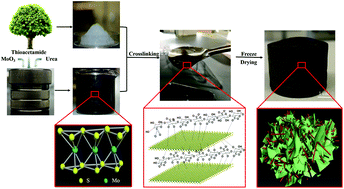
Thermally insulating materials, made from earth-abundant and sustainable resources, are highly desirable in the sustainable construction of energy efficient buildings. Cellulose from wood has long been recognized for these characteristics. However, cellulose can be a flammability hazard, and for construction this has been addressed via chemical treatment such as that with halogen and/or phosphorus, which leads to further environmental concerns. Fortunately, the structure of cellulose lends itself well to chemical modification, giving great potential to explore interaction with other compounds. Thus, in this study, cellulose nanofibers (CNFs) were nano-wrapped with ultrathin 1T phase molybdenum disulfide (MoS2) nanosheets via chemical crosslinking, to produce an aerogel. Thermal and combustion characterization revealed highly desirable properties (thermal conductivity k = 28.09 mW m−1 K−1, insulation R value = 5.2, limit oxygen index (LOI) = 34.7%, total heat release = 0.4 MJ m−2). Vertical burning tests also demonstrated excellent fire retardant and self-extinguishing capabilities. Raman spectra further revealed that MoS2 remained unscathed after 30 seconds of burning in a 1300 °C butane flame. Considering the inherently low density of this material, there is significant opportunity for its usage in a number of insulating applications demanding specific fire resistance properties.


 Please wait while we load your content...
Please wait while we load your content...