Appreciation of symmetry in natural product synthesis
Abstract
Covering: 2012 to June 2017
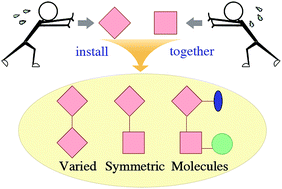
This review aims to show that complex natural product synthesis can be streamlined by taking advantage of molecular symmetry. Various strategies to construct molecules with either evident or hidden symmetry are illustrated. Insights regarding the origins and adjustments of these strategies as well as inspiring new methodological developments are deliberated. When a symmetric strategy fails, the corresponding reason is analysed and an alternative approach is briefly provided. Finally, the importance of exploiting molecular symmetry and future research directions are discussed.


 Please wait while we load your content...
Please wait while we load your content...