Acridone in a biological nanocavity: detailed spectroscopic and docking analyses of probing both the tryptophan residues of bovine serum albumin
Abstract
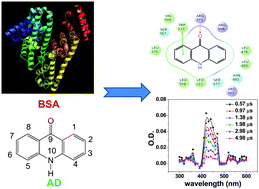
Spectroscopic and docking analyses reveal that acridone (AD) interacts with both the tryptophan (Trp) residues of bovine serum albumin (BSA) (both Trp 134 and Trp 212) in contrast to other organic ligands including other acridine derivatives which generally prefer to interact with Trp 212. The use of fluorescence spectroscopy, specifically the unusual time-resolved area normalized spectra depicting two isoemissive points with different times of evolution, confirms that AD “unusually” interacts with both the Trp residues present in the model protein. Upward curvature of the Stern Volmer plot suggests the interaction of AD with both the Trp residues present in varying microenvironments within BSA and possibly also indicates the denaturation of the protein. Ground state interaction of AD and BSA is explored using absorption spectroscopy, whereas strong perturbation in secondary and tertiary structures of the model protein on binding with the ligand is divulged from the observation of circular dichroism spectroscopy. Femtosecond fluorescence up-conversion kinetics implies that a photoinduced electron transfer reaction takes place from the Trp residue of the protein to AD, which has been authenticated using laser flash photolysis via identification of the radical ions. Binding as well as thermodynamic parameters associated with AD–BSA interaction are obtained from fluorescence studies. The prime deduction from the detailed spectroscopic and docking analyses is that AD initially interacts with Trp 212 present in the crevice of hydrophobic domain IIA of the protein and then perturbs the structure of BSA to bring about conformational changes such that it can gain access to Trp 134 housed in hydrophilic domain IB, which is possibly facilitated by hydrogen bonding.


 Please wait while we load your content...
Please wait while we load your content...