A rhodamine based turn-on chemosensor for Fe3+ in aqueous medium and interactions of its Fe3+ complex with HSA†
Abstract
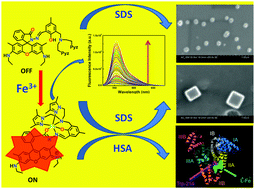
A novel hexa-coordinating rhodamine-based chemosensor, HL6, with N4O2 donor atoms, selectively and rapidly recognizes Fe3+ in the presence of all biologically relevant as well as toxic metal ions, numerous anions (except for F− and I−, which quench FI) and amino acids. The lower detection limit (110 nM) along with cytoplasmic cell imaging applications with negligible cytotoxicity provides a good opportunity towards in vitro/in vivo cell imaging of Fe3+ ions. Scanning electron microscopy (SEM) studies reveal a spherical microstructure for the free ligand, HL6, which melts upon increasing the applied voltage (≥2 kV); however, this is reduced in size in the presence of SDS, morphology remaining the same. [L6-Fe]2+ gives hexagonal rod-like microstructures due to π⋯π stacking extended in a one dimensional fashion, which, in the presence of SDS, turn into cubic microstructures. However, in the presence of HSA both HL6 and [L6-Fe]2+ agglomerated severely. A plot of FI (or r) vs. [SDS] at constant [L6-Fe2+] (20 μM) shows a rapid decrease in FI (or r); FI or r reaches a minimum at ∼2 mM SDS and then increases and becomes saturated at ≥8 mM SDS. The initial rapid decrease in FI or r with the increase in [SDS] arises due to the breakdown of the self-assembled microstructures of [L6-Fe]2+ formed probably through π⋯π stacking in pure aqueous solution in the presence of SDS (≤2 mM). The increase in FI or r with [SDS] beyond 2 mM may arise due to trapping of the [L6-Fe]2+ moiety inside the micellar cavity thereby giving it a thermodynamically rigid structure. Again, fluorescence anisotropy measured as a function of HSA concentration (0–70 μM) at a fixed concentration of HL6 and Fe3+ (20 μM each) at 555 nm showed a gradual increase in r with the increase in [HSA]. Site selective binding and molecular modeling studies revealed that [L6-Fe]2+ predominantly binds in the subdomain IIA of HSA by both hydrophobic and electrostatic forces. A circular dichroism (CD) study clearly points towards the reduction in α-helical organization of the protein and subsequent increase in the coiled structure upon binding with [L6-Fe]2+ indicating a small but definitive partial unfolding of the protein.


 Please wait while we load your content...
Please wait while we load your content...