Study of counter electrodes as an effective controlling factor of crystal orientation of TiO2 nanoarrays used as the anode in lithium-ion batteries†
Abstract
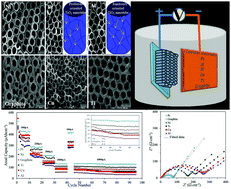
In this paper, we report the synthesis of titanium dioxide nanoarrays (TNAs) via an anodization process using different counter electrodes, including Pt, Ni, Ti, Cu, Al, and graphite. The synthesized TNAs are studied as anode materials for lithium ion batteries. The crystallographic structure and surface morphology of the TNAs are studied using X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM). Electrochemical tests of the TNA-Li half-cells are carried out using cyclic voltammetry (CV), charge/discharge analysis, and electrochemical impedance spectroscopy (EIS). These results show that the TNA morphologies and crystallography strongly depend on the type of counter electrode. The synthesis of TNAs using Pt and Al as a counter electrode leads to the highest and lowest crystal growth in the preferred 001 direction, respectively, in comparison with other counter electrodes. The TNAs anodized using a Pt counter electrode illustrate an acceptable cyclability and rate capability up to 100 cycles.


 Please wait while we load your content...
Please wait while we load your content...