A roll-to-roll process for multi-responsive soft-matter composite films containing CsxWO3 nanorods for energy-efficient smart window applications†
Xiao
Liang‡
a,
Chongshen
Guo‡
 d,
Mei
Chen
a,
Shumeng
Guo
b,
Lanying
Zhang
a,
Fasheng
Li
d,
Mei
Chen
a,
Shumeng
Guo
b,
Lanying
Zhang
a,
Fasheng
Li
 e,
Shaojun
Guo
e,
Shaojun
Guo
 *ac and
Huai
Yang
*a
*ac and
Huai
Yang
*a
aDepartment of Materials Science and Engineering, College of Engineering, Peking University, Beijing 100871, People's Republic of China. E-mail: yanghuai@pku.edu.cn; guosj@pku.edu.cn
bDepartment of Materials Physics and Chemistry, School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, People's Republic of China
cBIC-ESAT, College of Engineering, Peking University, Beijing 100871, China
dMicro- and Nanotechnology Research Center, Harbin Institute of Technology, Harbin 150080, China
eDepartment of Chemistry, Dalian Medical University, Dalian 116044, China
First published on 11th August 2017
Abstract
This work provides a roll-to-roll processed flexible multi-responsive smart film containing tungsten bronze nanorods and a phase-separated liquid crystal—polymer composite, which can reversibly control the passage of visible light in response to temperature, an electric field and near infrared light, and also screen the heat rays from 800 nm to 2500 nm.
Conceptual insightsSmart windows that have functionalities of shielding near infrared (NIR) rays and controllable visible light transmittance can not only conserve electric energy as a result of the conditioner operation, but also protect privacy and prevent indoor members from intensive light stimuli. Herein we present a novel roll-to-roll production of a multi-responsive soft-matter composite smart film that can not only control the passage of visible light by temperature, an electric field and NIR light, but also screen more than 95% of the NIR irradiation from 800 nm to 2500 nm. These excellent properties of the film were achieved by creating a compatible poly(vinylpyrrolidone) (PVP) tuning layer between tungsten bronze (CsxWO3) nanorods (NRs) and a polymeric syrup containing liquid crystals with a smectic A (SmA) to chiral nematic (N*) phase transition, followed by forming an elaborated–designed polymer structure within the film. Besides, the film also exhibits a wide working temperature range, excellent flexibility, high mechanical strength, long-term stability and large-area processability. The as-prepared film shows great potential for application as a smart energy-saving window, and the proposed strategy provides new insights into engineering novel hybrid inorganic–organic functional materials. |
Buildings account for at least 40% of energy use in most countries, and this energy consumption is still increasing rapidly, as the construction industry booms, especially in developing countries.1,2 Among all the components of a building, windows are often regarded as a less energy efficient building envelope with a larger maintenance requirement.3 The calculations show that more than 50% of the energy used in lighting, heating and cooling can be saved by deploying better control systems such as smart windows to control the influx of radiant heat transfer.4 Therefore, great efforts have been devoted to developing smart windows to replace the traditional way of sun-shading.5–15
Several aspects must be considered for smart windows to be used as an energy-efficient building envelope in areas where human inhabitants employ them. Of the utmost importance is their optical modulation range in the whole solar spectrum ranging from visible to NIR light (800–2500 nm).3,16,17 The invisible NIR light, especially the shorter wavelength range from 800 to 1500 nm, carries about 50% of the whole solar energy,18 and therefore the shielding performance of a smart window in this wavelength range is crucial for the purpose of improving the energy efficiency.19,20 Equally, the regulation of visible light transmittance is also desirable for the purposes of blocking the intensive light on scorching days from outside and protecting privacy.1,21 However, although our recent work demonstrated a broadband optical modulation within a flexible smart film,22 it is still a great challenge to combine the regulation of visible light transmittance and the shielding performance of NIR light from 800 nm to 2500 nm within a single material. Apart from optical modulation, other key factors including large-scale processability,22 long-term stability,23 good flexibility,24 high mechanical strength25 and functionality over a range of exterior temperatures2 should also be taken into account in material design for practical applications. Unfortunately, materials for smart windows integrated with all the above features have not been reported to the best of our knowledge.
Herein, we present a facile roll-to-roll production of an energy-efficient flexible smart film that can not only control the transmittance of visible light by temperature, electric fields and NIR light stimuli, but also screen more than 95% of NIR irradiation from 800 nm to 2500 nm. Our material for the smart film has a unique phase-separated polymer structure with a thickness of about 20 μm in which LCs with a smectic (SmA) to chiral nematic (N*) phase transition (PT-LCs) and tungsten bronze (CsxWO3) nanorods (NRs) are homogeneously dispersed. It is essentially a LC/polymer composite; unlike traditional polymer dispersed liquid crystal (PDLC) materials, which exhibit an opaque appearance in the off state,26–28 our smart film is transparent under normal conditions. Also, the high content of polymer in our material provides excellent mechanical strength, much higher than traditional polymer stabilized liquid crystal (PSLC) materials.29,30 Moreover, the polymeric syrup containing highly compatible organic and inorganic components allows our material to be facilely processed via roll-to-roll production and thus realize large-scale manufacturing. Finally, a simulated system was used to characterize the indoor temperature regulation performance of this multi-responsive smart composite material, showing that the as-made film was suitable for use as an energy-efficient smart window.
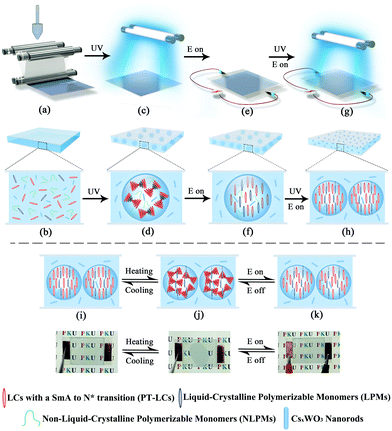
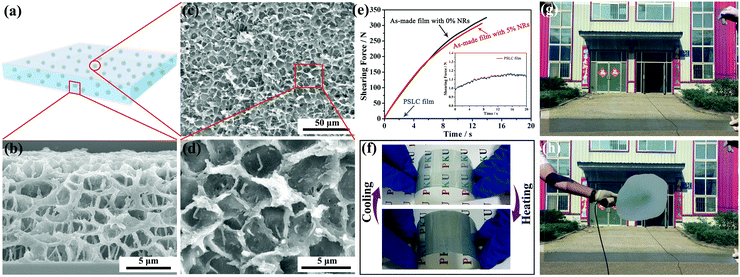
The procedures for making the smart film are illustrated in Scheme 1. First, a homogeneous polymeric syrup containing PT-LCs, liquid-crystalline polymerizable monomers (LPMs), non-liquid-crystalline polymerizable monomers (NLPMs) and CsxWO3 NRs were sandwiched between two plastic substrates via a roll-to-roll process, as shown in Scheme 1a and b. Then, in Scheme 1c, the film was irradiated under UV light for a short time to initiate a partial polymerization of the NLPMs and LPMs, leading to the formation of the phase-separated composite with a preliminary porous structure (Scheme 1d). In this step, the curing time should be precisely controlled within 90 seconds; otherwise, most of the LPMs would be consumed. After that, an electric field was applied across the film to homeotropically align the PT-LCs and LPMs (Scheme 1e and f). Meanwhile, a second-step of UV irradiation was carried out (Scheme 1g) to make the rest of the NLPMs and LPMs fully polymerized, forming oriented liquid-crystalline polymer networks (OLPNs) within the porous polymer matrix (Scheme 1h). After this two-step UV polymerization, a flexible multi-responsive energy-efficient smart film was prepared. When the environmental temperature is lower than the phase transition temperature of the PT-LCs, the PT-LCs in the SmA phase in the porous polymer are perpendicularly oriented to the substrate and stabilized by the OLPNs within the LC domains. The film exhibits a transparent state due to the well-matched refractive index between the PT-LCs and polymer. When the temperature surpasses the phase transition temperature of the PT-LCs, a focal-conic texture of the heat-induced N* phase of the PT-LCs is spontaneously formed, and the film becomes opaque due to the spatially varied refractive index between the PT-LCs and polymer. Also, this opaque state can be switched back into the transparent state either by decreasing the temperature into the SmA range (homeotropically aligned SmA phases would form again induced by the OLPNs) or by actively applying an electric field to homeotropically align the PT-LCs in the N* phase. Moreover, more than 95% of the NIR irradiation from 800 nm to 2500 nm in the solar spectrum can be effectively screened by the film due to the strong and broadband LSPR absorption of CsxWO3 NRs. Meanwhile, the absorbed NIR energy can be immediately transformed into heat to initiate the SmA to N* phase transition, meaning that the passage of visible light in our film can not only be controlled by temperature and electric fields, but also NIR light stimuli.
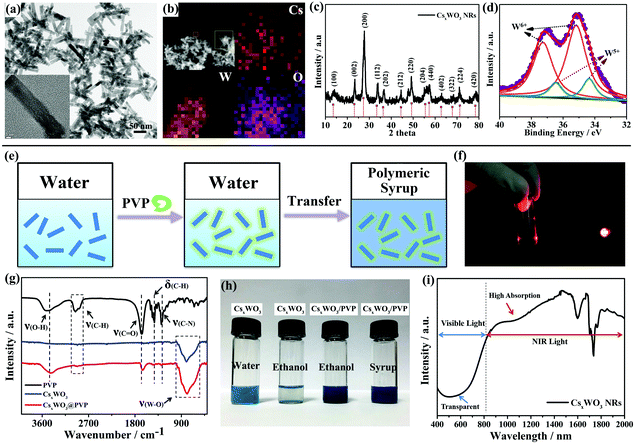
CsxWO3 NRs were synthesized using a previously reported method.31 They exhibit a rod-like morphology. The diameter and length of these NRs are 12 ± 2 nm and 68 ± 5 nm, respectively (Fig. 1a). Energy dispersive X-ray spectroscopy (EDS) mapping (Fig. 1b) demonstrates the existence of Cs, W, and O elements in the products, and the Cs/W atomic ratio is determined to be 0.32. The X-ray diffraction (XRD) pattern in Fig. 1c shows the hexagonal structure of Cs0.32WO3 (JCPDS No. 831334), without impurity peaks. XPS results suggest the mixed bonding state of tungsten element. The main peaks with a W 4f 5/2 at 37.3 eV and a W 4f 7/2 at 35.2 eV can be ascribed to W+6, while the second doublet with a lower binding energy at 34.4 and 36.5 eV is attributed to the W 4f 5/2 and W 4f 7/2 core levels from W+5. This mixed W+5 and W+6 is agreement with that of CsxWO3 tungsten bronze, expressed as CsxWx+5W1−x+6O3.26
The as-made CsxWO3 NRs were found to be well-dispersed in water because of their high zeta-potential value of −67.55 ± 0.92 mV; however, they aggregate spontaneously after being introduced into the polymeric syrup containing LCs and polymerizable monomers likely because of the compression of the surface electric double layer, which not only results in a loss of transparency in the visible region, but also detrimentally affects the NIR shielding performance of the CsxWO3 NRs, which resulted from a significant reduction in the surface electron density in large aggregates. Thus, to render these NRs well incorporated into this organic polymeric system, we promote an facile strategy by coating the surface of the CsxWO3 NRs with a layer of poly(vinylpyrrolidone) (PVP) as a tuning agent. The polar functional groups such as the amide of the coated PVP layer can make the interface between CsxWO3 NRs and the polymeric syrup highly compatible since the syrup containing LCs and polymeric monomers can be viewed as a solvent with high polarity.
As shown in Fig. 1e, the pristine CsxWO3 NR powders were first dispersed in deionized water under ultrasonication to form a homogeneous dispersion. After that, a trace amount of PVP aqueous solution was introduced into the dispersion under continuous stirring for 24 h to allow a full absorption of PVP onto the CsxWO3 NRs. Then, after being centrifuged and rinsed with deionized water, these PVP functionalized CsxWO3 (CsxWO3/PVP) NRs can be readily dispersed in the polymeric syrup and other organic solvents such as ethanol and acetone. The Tyndall effect in Fig. 1f indicates the existence of CsxWO3 NRs in the polymeric syrup.
To demonstrate the success of this PVP coating process, an FT-IR spectrum was used to measure the surface chemistry of the CsxWO3/PVP NRs. In Fig. 1g, five distinct peaks of PVP are observed, attributed to the stretch vibrational bands of ν(O–H) at around 3490 cm−1, the stretch vibrational bands of ν(C–H) at 2938–2854 cm−1, the stretch vibrational bands of ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 1655 cm−1, the flexural vibrational bands of δ(C–H) at 1423 cm−1 and the stretch vibrational bands of ν(C–N) at 1290 cm−1. In contrast, the unmodified CsxWO3 NRs exhibit no obvious peaks except the stretch vibrational bands of ν(W–O) at around 784 cm−1. After PVP coating, the distinct peaks of PVP were all detected for the CsxWO3/PVP NRs, indicating that the PVP coating process was successful. These CsxWO3/PVP NRs can be stable for at least one month in the polymeric syrup, while the unmodified CsxWO3 NRs would form large aggregates and be fully precipitated in polar solvents within half an hour (Fig. 1h). Most importantly, those CsxWO3/PVP NRs show intensive and broadband absorption in the NIR wavelength range from 800 nm to 2500 nm, but only a slight absorption in the visible light range 400 nm to 800 nm, exhibiting promising performance to be used as solar filters, as confirmed by absorption spectra in Fig. 1i.
O) at 1655 cm−1, the flexural vibrational bands of δ(C–H) at 1423 cm−1 and the stretch vibrational bands of ν(C–N) at 1290 cm−1. In contrast, the unmodified CsxWO3 NRs exhibit no obvious peaks except the stretch vibrational bands of ν(W–O) at around 784 cm−1. After PVP coating, the distinct peaks of PVP were all detected for the CsxWO3/PVP NRs, indicating that the PVP coating process was successful. These CsxWO3/PVP NRs can be stable for at least one month in the polymeric syrup, while the unmodified CsxWO3 NRs would form large aggregates and be fully precipitated in polar solvents within half an hour (Fig. 1h). Most importantly, those CsxWO3/PVP NRs show intensive and broadband absorption in the NIR wavelength range from 800 nm to 2500 nm, but only a slight absorption in the visible light range 400 nm to 800 nm, exhibiting promising performance to be used as solar filters, as confirmed by absorption spectra in Fig. 1i.
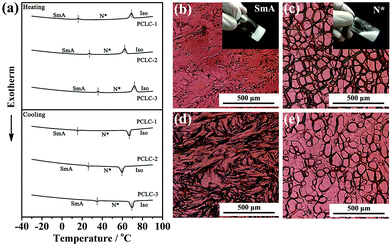
Obviously, the temperature range (TR) of PT-LCs is a crucial parameter in determining whether or not the as-made film is applicable for use over a range of exterior temperatures, since the TR of most PT-LC monomers is quite narrow. For example, the liquid-crystalline TR of 8CB (Cr 21.5 °C SmA 33.5 °C N 40.5 °C I) is only 19 °C. If used, the film would lose its functionality either below 21.5 °C or above 40.5 °C, which cannot be used in rigid environments. For practical applications, an ultra-wide liquid-crystalline TR is absolutely desirable. Here, our strategy for widening the TR was by mixing different LC monomers together to form a PT-LC mixture. The chemical structures of the LC monomers we used are provided in Scheme S1 (ESI†). Here, three PT-LC mixtures with different compositions are given. Differential scanning calorimetry (DSC) measurements in Fig. 2a indicate that they all exhibit an ultra-wide TR of more than 95 °C with a crystallization point below −30 °C and a clear point higher than 67 °C. Moreover, the SmA to N* phase transition temperature can be tuned from below 10 °C to above 40 °C (Fig. 2a), making the film much more designable to meet different preferences or requirements. Also, no impurity peaks were detected, indicating the good miscibility among the LC components. If not specified, we used PT-LC-2 (Cr < −30 °C SmA 31.5 °C N* 67.5 °C I) for next characterization and preparation. Fig. 2b and c show the typical textures of the SmA phase and the N* phase for PT-LC-2 at 25 °C and 35 °C, respectively. Fig. 2d and e show coexistent textures of SmA and N* phases during the heating and cooling processes, respectively. The inset in Fig. 2b indicates the high viscosity of the SmA phase, while the inset in Fig. 2c shows a drastic decrease of the viscosity of the N* phase compared with the SmA phase.
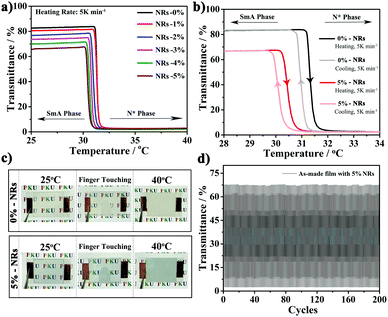
Smart films containing CsxWO3/PVP NRs were prepared by the step-wise polymerization in Scheme 1. Fig. 3a shows the temperature-dependent transmittance for each film with different amounts of CsxWO3/PVP from 0% to 5 wt% at 550 nm. All the as-made films exhibit a transmittance transition from highly transparent to opaque as the temperature surpasses the phase transition temperature of PT-LC-2. We further tested the thermo-optical properties of the films with 0% and 5 wt% CsxWO3/PVP NRs during the heating and cooling processes, respectively. As shown in Fig. 3b and c, the transmittance of visible light in both of the as-made films can be reversibly changed by controlling the temperature. The decreasing trend of transmittance in the transparent state of the films with more CsxWO3/PVP NRs (Fig. 3a and b) can be attributed to a slight absorption of CsxWO3/PVP NRs in the visible range, but it was acceptable as 5 wt% CsxWO3/PVP NRs were incorporated. Furthermore, to test the stability and reversibility of the as-made film, the transmittance of our film at 550 nm was tested during a heating–cooling cycle 200 times. As shown in Fig. 3d, the transmittance of the transparent and opaque states almost reached the same values as their previous ones, demonstrating the total reversibility and stability of our film.
This reversible change in transmittance can be attributed to the formation of OLPNs crosslinked between LCMs during the second-step UV polymerization within the porous polymer matrix, as confirmed by scanning electron microscopy (SEM) from both top (Fig. 4c and d) and side views (Fig. 4b). Note that before SEM observation, all the as-prepared films were dipped in cyclohexane for 15 days to fully extract the LC molecules. During the SmA to N* phase transition, the OLPNs within the LC domains induced the orientations of the SmA phases to be homeotropically aligned, making the film become transparent. Otherwise, the SmA phase would form a focal-conic texture, and the film would still be opaque after phase transition. Moreover, the high content of the porous polymer matrix provides both excellent mechanical strength and flexibility to the film. We tested the shearing strength of the as-made films containing 0% and 5 wt% CsxWO3/PVP NRs (Fig. 4e). The shearing force of the as-made film with 5 wt% CsxWO3/PVP NRs reached as high as 306.9 N, comparable to the as-made film without CsxWO3/PVP NRs of the same size, and ∼255 times higher than the traditional PSLC films. Additionally, we also tested the flexibility of the film. In Fig. 4f, the excellent thermo-optical properties of the film were well preserved under folding or bending. Besides, using roll-to-roll production, the film can be manufactured on a large-scale with a size of 1.0 m × 1.5 m (Fig. 4g).
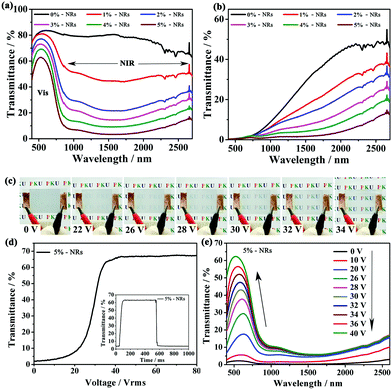
Due to the strong and broadband LSPR absorption of CsxWO3/PVP NRs in the NIR region from 800 nm to 2500 nm, the NIR irradiation from the solar spectrum can be effectively rejected. We systematically investigated the Vis-NIR transmittance spectra of our film containing different amounts of CsxWO3/PVP NRs in both transparent and opaque states. Obviously, as shown in Fig. 5a and b, the NIR shielding performance of the as-made films was enhanced as more CsxWO3/PVP NRs were introduced. In particular, more than 95% of the NIR irradiation could be screened by incorporating 5 wt% CsxWO3/PVP NRs in both transparent and opaque states. The phase transition from LCs mainly altered the transmittance of the visible light in the film, while the CsxWO3/PVP NRs acted as solar filters to reject NIR light.
The electro-optical properties of the film were also investigated since LCs are electric field responsive materials. As shown in Fig. 5c, the film containing 5 wt% CsxWO3/PVP NRs could be actively and dynamically switched from the light scattering to transparent state under an applied voltage of ∼34 V. Fig. 5d shows the voltage dependence of the transmittance of the film. The result reveals that the threshold voltage (Vth) and saturation voltage (Vsat) of the film are 22.3 V and 34.2 V, respectively. Here, the Vth and Vsat values are defined as the voltages required for the transmittance to reach 10% and 90%, respectively. The inset in Fig. 5d shows that the response time of the film is ∼100 ms. Vis-NIR spectra of the film (Fig. 5e) indicates that the applied voltage mainly increased the transmittance in the visible region, but the NIR light could still be effectively rejected due to the absorption of CsxWO3/PVP NRs in the film.
Apart from strong LSPR absorption in the NIR region, the CsxWO3/PVP NRs in the as-made film could also act as nano-heaters to transform the absorbed NIR energy into heat, and initiate the SmA to N* phase transition. In this way, the passage of visible light can also be controlled by NIR light stimuli. To confirm the NIR light-responsive property, the films with different contents (0–5 wt%) of CsxWO3/PVP NRs were irradiated using a NIR lamp (PHILIPS, R125) at the same power density. The temperature variations of the films were tracked using a thermographic camera (Fig. 6a). Thermographic images were taken at different time intervals of 0 s, 5 s, 15 s, 35 s and 55 s. As shown in Fig. S2 (ESI†) and Fig. 6b, the temperature increased more rapidly with a higher content of CsxWO3/PVP NRs, indicating that the CsxWO3/PVP NRs within the films could rapidly and effectively convert NIR energy into heat under NIR light exposure. Furthermore, we also measured the transmittance (550 nm) of the films at different exposure time intervals (Fig. 6c). The film without CsxWO3/PVP NRs shows no obvious optical change under NIR light exposure. However, the transmittance of the films containing CsxWO3/PVP NRs underwent a sharp decrease during the measurements. In particular, the film with 5 wt% CsxWO3/PVP NRs could change from transparent to light-scattering within 5 seconds, exhibiting a fast response time under NIR exposure.
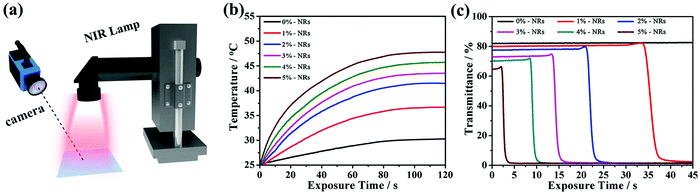 | ||
| Fig. 6 (a) Schematic illustration of the simulated experiment. (b) Temperature and (c) transmittance variations of the films with different amounts of CsxWO3/PVP NRs under NIR light exposure. | ||
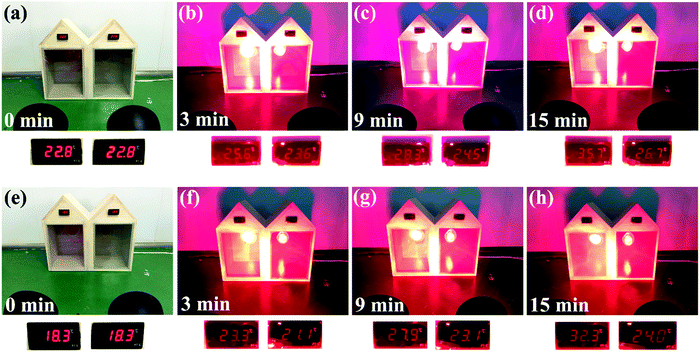
A comparative study was carried out to compare the thermoregulation effects of the as-made smart films containing 5 wt% CsxWO3/PVP NRs and pure PET films (Fig. 7a–d). After a continuous simulated solar irradiation for 15 min, the temperature in the left model house attached with a pure PET film significantly increased from 22.8 °C to 35.7 °C, while the temperature in the right house attached with an as-made smart film containing 5 wt% CsxWO3/PVP NRs changed from 22.8 °C to only 26.7 °C, indicating that the as-made smart film containing 5 wt% CsxWO3/PVP NRs exhibited excellent performance in keeping the indoor temperature balanced and was applicable as an energy-saving smart window. Furthermore, we also compared the thermoregulation effects of the as-made smart film containing 5 wt% CsxWO3/PVP NRs with the smart film without CsxWO3/PVP NRs. As shown in Fig. 7e–h, the indoor temperature of the left house attached with an as-made smart film containing 0% CsxWO3/PVP NRs noticeably went up from 18.3 °C to 32.3 °C after simulated solar radiation for 15 min, while that of the right house attached with an as-made smart film containing 5 wt% CsxWO3/PVP NRs increased to only 24 °C during the same period. This result indicates that the CsxWO3/PVP NRs within the as-made films play a key role in the shielding performance for heat rays.
Conclusions
In summary, we present a novel roll-to-roll production of a multi-responsive soft-matter composite smart film that can not only control the passage of visible light by temperature, electric fields and NIR light, but also screen 95% of the NIR solar irradiation from 800 nm to 2500 nm. These excellent properties of the film were achieved by creating a compatible PVP tuning layer between CsxWO3 NRs and a polymeric syrup containing PT-LCs, followed by forming an elaborated–designed polymer structure within the film. The transmittance of the film in the visible region can be not only reversibly changed between 67% and 1.5% in response to temperature and NIR light stimuli accompanied by a SmA–N* phase transition, but also actively and dynamically regulated by applying an electric field to homeotropically align the LCs. Moreover, more than 95% of the NIR irradiation can also be effectively blocked due to the strong LSPR absorption of CsxWO3 NRs well-dispersed within the composite. Besides, the film also exhibits a wide working temperature range, excellent flexibility, high mechanical strength, long-term stability and large-area processability. The as-prepared film shows great potential for application as a smart energy-saving window, and the proposed strategy provides new insights into engineering novel hybrid inorganic–organic functional materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) (Grant No. 51333001, 51573006, 51561135014, 51671003, 51572059 and 51372029), the Major Project of Beijing Science and Technology Program (Grant No. Z121100006512002), National Basic Research Program of China (No. 2016YFB0100201), and the Sino-American Cooperative Project of the Chinese Ministry of Science and Technology (Grant No. 2013DFB50340).Notes and references
- D. Ge, E. Lee, L. Yang, Y. Cho, M. Li, D. S. Gianola and S. Yang, Adv. Mater., 2015, 27, 2489 CrossRef CAS PubMed.
- H. Khandelwal, A. P. H. J. Schenning and M. G. Debije, Adv. Energy Mater., 2017, 7, 1602209 CrossRef.
- R. Baetens, B. P. Jelle and A. Gustavsen, Sol. Energy Mater. Sol. Cells, 2010, 94, 87 CrossRef CAS.
- N. Deforest, A. Shehabi, J. O'Donnell, G. Garcia, J. Greenblatt, E. S. Lee, S. Selkowitz and D. J. Milliron, Build. Environ., 2015, 89, 107 CrossRef.
- H. Shin, S. Seo, C. Park, J. Na, M. Han and E. Kim, Energy Environ. Sci., 2016, 9, 117 CAS.
- Y. Gao, C. Cao, L. Dai, H. Luo, M. Kanehira, Y. Ding and Z. L. Wang, Energy Environ. Sci., 2012, 5, 8708 CAS.
- M. J. Powell, R. Quesada-Cabrera, A. Taylor, D. Teixeira, I. Papakonstantinou, R. G. Palgrave, G. Sankar and I. V. Parkin, Chem. Mater., 2016, 28, 1369 CrossRef CAS.
- M. J. Debije, Adv. Funct. Mater., 2010, 20, 1498 CrossRef CAS.
- H. Y. Lee, Y. Cai, S. Bi, Y. N. Liang, Y. Song and X. M. Hu, ACS Appl. Mater. Interfaces, 2017, 9, 6054 CAS.
- Z. Xie, X. Jin, G. Chen, J. Xu, D. Chen and G. Shen, Chem. Commun., 2014, 50, 608 RSC.
- D. K. Yang and Q. Li, Polymer stabilized cholesteric liquid crystal for switchable windows, Liquid Crystals Beyond Displays, John Wiley & Sons, Hoboken, 2012, p. 505 Search PubMed.
- K. G. Gutierrez-Cuevas, L. Wang, Z. Zheng, H. K. Bisoyi, G. Li, L. S. Tan, R. A. Vaia and Q. Li, Angew. Chem., Int. Ed., 2016, 55, 13090 CrossRef CAS PubMed.
- L. Wang, H. K. Bisoyi, Z. Zheng, K. G. Gutierrez-Cuevas, G. Singh, S. Kumar, T. J. Bunning and Q. Li, Mater. Today, 2017, 20, 230 CrossRef CAS.
- X. Liang, S. Guo, M. Chen, C. Li, Q. Wang, C. Zou, C. Zhang, L. Zhang, S. Guo and H. Yang, Mater. Horiz., 2017 10.1039/C7MH00224F.
- Y. Gao, S. Wang, H. Luo, L. Dai, C. Cao, Y. Liu, Z. Chen and M. Kanehira, Energy Environ. Sci., 2012, 5, 6104 CAS.
- A. Llordés, G. Garcia, J. Gazquez and D. J. Million, Nature, 2013, 500, 323 CrossRef PubMed.
- Y. Zhou, Y. Cai, X. Hu and Y. Long, J. Mater. Chem. A, 2014, 2, 13550 CAS.
- Y. Sang, Z. Zhao, M. Zhao, P. Hao, Y. Leng and H. Liu, Adv. Mater., 2015, 27, 363 CrossRef CAS PubMed.
- Z. Yu, Y. Yao, J. Yao, L. Zhang, Z. Chen, Y. Gao and H. Luo, J. Mater. Chem. A, 2017, 5, 6019 CAS.
- Y. Gao, W. Yao, J. Sun, H. Zhang, Z. Wang, L. Wang, D. Yang, L. Zhang and H. Yang, J. Mater. Chem. A, 2015, 3, 10738 CAS.
- J. Murray, D. Ma and J. N. Munday, ACS Photonics, 2016, 4, 1 CrossRef.
- G. Cai, P. Darmawan, X. Cheng and P. S. Lee, Adv. Energy Mater., 2017, 7, 1602598 CrossRef.
- Y. Wang, E. L. Runnerstrom and D. J. Milliron, Annu. Rev. Chem. Biomol. Eng., 2016, 7, 283 CrossRef CAS PubMed.
- K. Wang, H. Wu, Y. Meng, Y. Zhang and Z. Wei, Energy Environ. Sci., 2012, 5, 8384 CAS.
- R. B. Goldner, T. Haas, K. K. Wong and G. Seward, US Pat., 4832463, 1989 Search PubMed.
- D. A. Higgins, Adv. Mater., 2000, 12, 251 CrossRef CAS.
- F. Kuschel, L. Hartmann and M. Bauer, Liq. Cryst., 2011, 38, 325 CrossRef CAS.
- J. K. Srivastava, R. K. Singh, R. Dhar and S. Singh, Liq. Cryst., 2011, 38, 849 CrossRef CAS.
- H. Kikuchi, M. Yokota, Y. Hisakado, H. Yang and T. Kajiyama, Nat. Mater., 2002, 1, 64 CrossRef CAS PubMed.
- I. Dierking, Adv. Mater., 2000, 12, 167 CrossRef CAS.
- C. Guo, S. Yin, L. Huang, L. Yang and T. Sato, Chem. Commun., 2011, 47, 8853 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nh00105c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |