Altered levels of circulating cytokines and microRNAs in lean and obese individuals with prediabetes and type 2 diabetes
Yury O.
Nunez Lopez
a,
Gabriella
Garufi
a and
Attila A.
Seyhan
*abc
aTranslational Research Institute for Metabolism and Diabetes, Florida Hospital, 301 East Princeton St., Orlando, FL 32804, USA. E-mail: Attila_seyhan@yahoo.com; Yury.Nunez-Lopez@flhosp.org; Tel: +617-682-2954
bSanford | Burnham Medical Research Institute, Orlando, FL, USA
cMassachusetts Institute of Technology, Chemical Engineering Department Cambridge, MA, USA
First published on 14th November 2016
Abstract
Today obesity and type 2 diabetes (T2D) have both reached epidemic proportions. However, our current understanding of the primary mechanisms leading to these diseases is still limited due to the complex multifactorial nature of the underlying phenomena. We hypothesize that the levels of specific cytokines and miRNAs vary across the diabetes spectrum and unique signatures associated with them may serve as early biomarkers of the disease and provide insights into respective pathogenetic mechanisms. In this study, we measured the circulating levels of cytokines and microRNAs (miRNAs) in lean and obese humans with prediabetes (n = 21), T2D (n = 17), and healthy controls (n = 20) (ORIGINS trial, NCT02226640). Data were analyzed by fitting linear models adjusted for confounding variables (BMI, age, and gender in the diabetes context and age, gender, and diabetes status in the obesity context) and implementing nonparametric randomization-based tests for statistical inference. Group differences and correlations (r > 0.3) between variables with P < 0.05 were considered significant. False discovery rates (FDR) correcting for multiple testing were calculated using the Benjamini–Hochberg correction. We found a number of circulating cytokines and miRNAs deregulated in subjects with obesity, prediabetes, and T2D. Specifically, cytokines IL-6, IL-8, IL-10, IL-12, and SFRP4, as well as miRNAs miR-21, miR-24.1, miR-27a, miR-28-3p, miR-29b, miR-30d, miR-34a, miR-93, miR-126, miR-146a, miR-148, miR-150, miR-155, and miR-223, significantly changed across the diabetes spectrum, and were associated with measures of pancreatic islet β cell function and glycemic control, among others. Notably, SFRP4 was the only studied cytokine that was significantly associated with obesity, prediabetes, and T2D, which underscores the important role of this molecule during disease development and progression. Our data suggest that changes in circulating miRNAs and cytokines may have clinical utility as biomarkers of prediabetes.
Introduction
Although obesity is an established risk factor for metabolic diseases including type 2 diabetes (T2D), our current understanding of the primary mechanisms leading to T2D is still limited. It is known that tissue inflammation mediated by the stimulation of different immune signaling molecules such as cytokines plays an important role in the pathogenetic mechanisms of diabetes. In particular, inflammatory processes contribute to glucotoxicity, lipotoxicity, oxidative stress, and endoplasmic reticulum stress during the development of the disease.1 Elevated systemic concentrations of proinflammatory cytokines/chemokines and downregulation of anti-inflammatory adiponectin, for example, lead to chronic subclinical inflammation that is frequently associated with the development of insulin resistance, pancreatic islet β cell dysfunction, and eventually T2D.2 Diabetes is also characterized by upregulation of specific anti-inflammatory proteins,1 which may be part of compensatory mechanisms and/or mechanisms involved in the regulation of specific immune cell subsets. However, the activation state of the immune system depends on complex interactions among multiple genetic and environmental factors3 that contribute to the heterogeneity of T2D. Obesity, for example, is an important risk factor for diabetes. For example, the influx of macrophages and T cells into adipose tissues during obesity causes the release of proinflammatory mediators that cause insulin resistance.4 Obesity-induced chronic low-grade inflammation and the development of a metabolic syndrome have been suggested to qualify under the established criterion of “autoinflammatory disease cluster”.4 Autoinflammation is defined as a self-directed inflammatory process, “whereby local factors at sites predisposed to disease lead to activation of innate immune cells, including macrophages and neutrophils, with resultant target tissue damage”.5Cytokines are well-known modulators of inflammatory and immune responses, and microRNAs (miRNAs) are well-known small (18–22 nucleotides), non-protein coding RNAs that modulate the differentiation, growth, apoptosis and proliferation of cells by interfering with protein synthesis either by inducing mRNA degradation or repressing translation.6–8 It is estimated that miRNAs regulate the expression of more than 60% of protein-coding genes.9 miRNAs have been shown to play key roles in the regulation of a broad spectrum of physiological and pathophysiological processes including obesity, metabolic dysfunction, diabetes, and aging, among others.10–14 Because the miRNA species in circulation may reflect the activation state of circulating cells or tissue injury in response to disease states, circulating miRNAs are also becoming increasingly recognized as powerful biomarkers for human diseases.15–19 Altered levels of circulating miRNAs have been reported in a variety of disease states including obesity, metabolic dysfunction, and diabetes.10
Importantly, miRNAs have been recently suggested to be critical components of complex regulatory networks in immune responses.20 In addition to directly regulating specific cytokines by interacting with miRNA-binding sites in the mRNA 3′UTR regions,21,22 miRNAs indirectly regulate cytokines by interfering with the recognition of AU-rich elements (AREs) by ARE-binding proteins and/or regulating the expression of the latter.20,23 The crosstalk between cytokines and miRNAs has also been demonstrated.24 Barbagallo et al. showed that murine pancreatic αTC1-6 and βTC1 cells are subjected to differential activity of miR-296-3p and miR-298-5p and that these differences accounted, at least in part, for the higher resistance of pancreatic islet α cells to apoptotic death induced by proinflammatory cytokines.24 This evidence has a direct impact on our mechanistic understanding of β cell loss during diabetes development. Another important immunologic role of miRNAs is in modulating M1–M2 macrophage phenotypic polarization, supported by growing evidence linking excessive or impaired macrophage-driven inflammatory responses with the deregulation of miRNAs.25
In this study, we characterized a panel of circulating cytokine and miRNA profiles in lean and obese humans with prediabetes and T2D to identify early biomarkers of diabetes development across the spectrum of glucose tolerance and to gain insights into potential mechanisms of T2D development and/or progression. We identified distinctive cytokine and miRNA signatures with early biomarker potential and discuss the potential mechanistic insights at the molecular regulatory level.
Methods
Research design and subjects
All procedures were approved by the Translational Research Institute for Metabolism and Diabetes (TRI-MD)/Florida Hospital (FH) Institutional Review Board (IRB). Informed consent was obtained from all volunteers before initiation of the study. The study cohort included 60 participants from the ORIGINS trial (NCT02226640): 20 healthy controls (11 lean + 9 with obesity), 21 with prediabetes (10 lean + 11 with obesity), and 17 with T2D (2 lean + 15 with obesity). The groups were classified as per ADA guidelines:26 healthy subjects (fasting glucose <100 mg dl−1), subjects with prediabetes [either 5.7 ≤ HbA1C < 6.5, or impaired fasting glucose (100 mg dl−1 ≤ fasting glucose < 126 mg dl−1), and subjects with diabetes (fasting glucose ≥ 126 mg dl−1, or HbA1C ≥ 6.5)]. Overweight/“with obesity” status was considered when the subject's BMI ≥ 25. The inclusion and exclusion criteria of the subjects were described previously (NCT02226640).Clinical and metabolic measurements
Anthropometric measures were performed according to standardized protocols. Body composition was measured using a GE Lunar iDEXA whole-body scanner (GE, Madison, WI). Fasting blood samples were obtained and subjects underwent a 2-hour 75 g oral glucose tolerance test (OGTT). Plasma glucose concentrations were measured using the glucose oxidase method with a YSI 2300 STAT Plus Analyzer (YSI Life Sciences, Yellow Springs, OH). Plasma insulin and C-peptide concentrations were determined using the MSD human insulin assay kit and C-peptide kit, respectively (MSD, Rockville, MD). HbA1c levels were measured using a Cobas Integra 800 Analyzer (Roche, Basel, Switzerland). The β cell function was assessed by calculating HOMA-B, the insulinogenic index [ΔIns-30′/ΔGluc-30′] and the insulin and c-peptide areas under the curve (AUC) in response to OGTT. Insulin activity was assessed by calculating HOMA-IR and the Quicki indices as described elsewhere.Enzyme-linked immunosorbent assay
The levels of a panel of serum cytokines IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF-α were measured using MSD's V-PLEX Proinflammatory Panel1 (human) kit and a Sector 2400 imager following the manufacturer's instructions. Serum SFRP4 was measured using the ELISA kit for human SFRP4 (Biomatik, Wilmington, DE) following the manufacturer's instructions. ELISA plates were analyzed on a Synergy 2 Multi-Mode Reader (Biotek, Winooski, VT). Intra- and inter-assay reproducibility in replicate assays for pro-inflammatory cytokines were CV: <7.0% (intra-plate) and CV: <15% (inter-plate) and for SFRP4 was CV: ≤10%.Total RNA extraction of circulating miRNA and qRT-PCR
Total RNA was extracted using a miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Real-time PCR was performed in triplicate using Life Technologies™ kits (Thermo Fisher Scientific, Waltham, MA) and following the manufacturer's instructions. In short, reverse transcription was done using a TaqMan® MicroRNA Reverse Transcription Kit, cDNA was pre-amplified through 12 cycles using the TaqMan® PreAmp Master Mix, and the qRT-PCR reaction was performed using TaqMan® Universal Master Mix II and TaqMan® MicroRNA assay. A diabetes-related human miRNA panel including miR-15a, miR-21, miR-24.1, miR-25, miR-27a, miR-28-3p, miR-29a, miR-29b, miR-30d, miR-34a, miR-93, miR-138, miR-146a, miR-148a, miR-150, miR-152, miR-155, miR-181a, miR-199a, miR-223, miR-320, miR-326, and miR-376 was used. qRT-PCR reactions generating raw Ct values greater than 35 were not included in the analysis (those data points were reassigned as NA values). The −ΔΔCt method was used for the calculation of relative expression values. The first normalization step adjusted the raw Ct values relative to the recovered levels of spike-in cel-miR-39 (to adjust for the variability in RNA extraction efficiency per sample). The second normalization step subtracted the geometric means of three endogenous control miRNAs: miR-191, miR-423-3p, and miR-451. These normalized −ΔΔCt data, equivalent to a log fold change (log FC) relative to the geometric mean of the endogenous control levels, were used for statistical analysis.Statistical analysis
For statistical inference, randomization tests27 were implemented in the R 3.3.1 environment.28 Imbalances in cohort demographic and clinical characteristic measures were assessed using the Monte Carlo Kruskal–Wallis test (Box 1) using the independence_test() function in the coin package, a valuable tool for COnditional INference analysis.29 This function provides a general framework for testing statistical independence and is based on the flexible conceptual framework for conditional inference procedures proposed by Strasser and Weber.30 A comprehensive description of the software implementation can be found in ref. 29. Differential abundance of circulating cytokines and miRNAs was assessed by multiple comparisons among groups, with subjects grouped either by diabetes status (diabetes context analysis) or by obesity status (obesity context analysis). The interaction analysis of diabetes status and obesity status was not possible due to the limited number of lean T2D subjects in our cohort. In the diabetes context analysis, cytokine and miRNA data were modeled as a function of the diabetes status (an independent variable with 3 levels: Healthy, Prediabetes, and T2D) adjusted for BMI, age, and gender. To gain insights into the obesity contribution, we tested an alternative model where each cytokine or miRNA was modeled as a function of the obesity status (an independent variable with 2 levels: Lean and Obese), adjusted for diabetes status, age and gender. The statistical metric used to measure the effect of interest in the observed one thousand iterations of the randomization test was the standardized statistic calculated using the independence_test() function (Box 2). To generate the standardized statistics for all pairwise comparisons between levels of the dependent variables, conditional nonparametric tests (equivalent to the Kruskal–Wallis test for the diabetes context analysis that included three “diabetes status” levels and to the Wilcoxon–Mann–Whitney test for the obesity context analysis that included two “obesity status” levels) were performed via a rank transformation of the response variable, followed by a Tukey's all-pairwise comparison via the specification of the model and contrast matrices for the dependent variables as shown in Box 2. Our specific formulas for this part of the analysis were “response_variable ∼ Group_Diabetes + Age + Gender + BMI” for the diabetes context analysis and “response_variable ∼ Group_Obesity + Age + Gender + Group_Diabetes” for the obesity context analysis. After extracting the standardized statistics for real data comparisons (the observed statistics) and for one thousand iterations of the test using randomly permuted data (which generates an approximate distribution of the standardized statistic), a two-sided P value can be calculated by determining the frequency of the absolute value of the calculated random statistics being greater than or equal to the absolute value of the observed statistic for a particular comparison. Partial correlations adjusting for BMI, age, and gender (in the diabetes context analysis) and adjusting for age, gender, and diabetes status (in the obesity context analysis) were calculated using the ppcor package.31 Group differences and correlations (r > 0.3) between variables with P < 0.05 were considered significant. Weak correlations with r < 0.3 were filtered out of the analysis and considered nonsignificant, independently of the corresponding P value. False discovery rates (FDR) correcting for multiple testing were calculated using the Benjamini–Hochberg correction.32|
Box 1. Implementing an univariate Monte Carlo Kruskal–Wallis test to assess the demographic and clinical characteristics of the study cohort.
## The inference test can be specified using the independence_test() function in the coin package: model = independence_test (response_variable ∼ Group_Diabetes, data = my_demographics_data_frame, ytrafo = rank_trafo, teststat=“quadratic”, distribution = approximate (B = 10000)) ## The global P value can be extracted with the pvalue() function: pvalue.global = pvalue (model) |
|
Box 2. Implementing a multivariate Kruskal–Wallis test with multiple comparisons
## The model and the inference tests for all pairwise comparisons can be specified using the independence_test() function in the coin package: model = independence_test (formula = my_specific_formula, data = my_data_frame, ytrafo = function (data) trafo(data, numeric_trafo = rank), xtrafo = function (data) trafo(data, factor_trafo = function(x) model.matrix(∼x − 1)%*% t(contrMat(table(x), “Tukey”))), teststat = “quadratic”) ## The standardized statistics can be extracted by using the statistic() function: stats.std = statistic (model, type = “standardized”) |
Results
Demographic and clinical characteristics of the study cohort
The subject characteristics of the study cohort are detailed in Table 1. Age presents a significant imbalance among subjects with obesity. However, the potential confounding effect of age is adjusted for in the models used for differential abundance and partial correlation analyses. HbA1c, fasting glucose, and glucose AUC were significantly imbalanced among the distinct groups, as expected by the study design. Several indices of insulin resistance and sensitivity showed significant differences mainly among the obese subjects, while the MATSUDA index marginally changed in the lean population.| Lean subjects | Subjects with obesity | |||||||
|---|---|---|---|---|---|---|---|---|
| Healthy | Prediabetes | T2D | P valuea | Healthy | Prediabetes | T2D | P valuea | |
| a P values were calculated using the Monte Carlo Kruskal–Wallis test, implemented in R using the coin package. Data are presented as median with 95% confidence interval of the median (in parenthesis). | ||||||||
| Anthropometrics & others | ||||||||
| Gender (female/male) | 8F/3M | 6F/4M | 1F/1M | 0.8415 | 7F/2M | 5F/6M | 5F/10M | 0.1139 |
| Age (years) | 32 (23, 67.5) | 42.5 (22.7, 58.6) | 41 (30.5, 51.5) | 0.5735 | 34 (19.4, 54) | 42 (35, 62.2) | 51 (36.4, 66.6) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[3 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0033_0332.gif) ![[5 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0035_0332.gif) ![[8 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0038_0332.gif)
|
| BMI (kg m−2) | 21.8 (20.3, 24.7) | 21.7 (18.8, 24.4) | 23.1 (22.3, 23.9) | 0.5908 | 35 (26.5, 37.4) | 35.1 (31.5, 52.7) | 36.5 (30.8, 52.1) | 0.5564 |
| Height (cm) | 163.9 (156.4, 179.9) | 169.2 (153.2, 183.4) | 171.1 (164.9, 177.2) | 0.7850 | 158.9 (150.4, 176.7) | 177.2 (154.3, 193) | 172.8 (154.8, 181.8) | 0.0793 |
| Weight (kg) | 63.8 (53, 74.7) | 56.6 (49.8, 76.4) | 67.5 (64.8, 70.2) | 0.6500 | 85.9 (77.9, 104.8) | 104.8 (81.2, 186.5) | 102 (81.7, 164) | 0.2576 |
| Waist circumference (cm) | 78.5 (68, 82.4) | 75.9 (69.2, 86) | 79.7 (79.2, 80.2) | 0.1249 | 105 (86.9, 120.3) | 111.2 (101, 154.2) | 116.8 (93.9,150.8) | 0.6172 |
| DXA_Fat% | 32 (16.5, 38.8) | 29.5 (16.1, 38.2) | 20.7 (19.4, 22) | 0.3063 | 48.5 (31.4, 49.9) | 43.3 (30.8, 55.9) | 46.3 (34.4, 53.2) | 0.9693 |
| Plasma lipids | ||||||||
| HDL | 68 (44.8, 90.2) | 63 (46, 116.4) | 64.5 (64, 65) | 0.4024 | 41 (35.2, 64.4) | 41 (31.2, 51) | 41 (27, 74) | 0.7450 |
| LDL | 105 (58.5, 163) | 135.5 (63, 155.1) | 91 (84.3, 97.7) | 0.0749 | 97 (67.2, 151.8) | 110 (89.3, 150.8) | 92 (45.4, 159.2) | 0.2810 |
| Triglycerides | 88 (48, 167.2) | 76 (40.1, 106.6) | 58.5 (48.5, 68.5) | 0.8818 | 120 (68.4, 238.8) | 115 (57.5, 441.5) | 171 (86.6, 290.8) | 0.2930 |
| OGTT-related measures | ||||||||
| HbA1c (%) | 5.3 (4.9, 5.6) | 5.8 (5.3, 6.2) | 6.1 (6, 6.2) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[1 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0031_0332.gif)
|
5.4 (5, 5.6) | 6 (5.6, 6.2) | 6.6 (6.1, 7.5) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif)
|
| Fasting glucose (mg dL−1) | 84.9 (71, 95.4) | 90 (78.7, 106.3) | 115.2 (91.4, 139.1) | 0.7688 | 88.2 (83.6, 97.6) | 103 (93.5, 110.9) | 126 (109.6, 167.6) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[3 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0033_0332.gif) ![[7 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0037_0332.gif)
|
| Glucose AUC | 14551.9 (12577.7, 17217.8) | 16466.7 (14123.6, 20554.1) | 16357.5 (10324, 22391) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0032_0332.gif) ![[8 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0038_0332.gif) ![[6 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0036_0332.gif)
|
16222.1 (13481.3, 17125.1) | 18942.7 (15271.4, 25810.1) | 25816 (19586.8, 33487) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif)
|
| Fastin insulin | 1.2 (0.8, 2.7) | 1.3 (0.8, 2.1) | 1.5 (0.5, 2.4) | 0.9198 | 3.9 (1.5, 14) | 11.1 (4, 23.3) | 9.2 (1.5, 31.5) | 0.7905 |
| Insulin AUC | 2010.9 (1030.2, 5511.4) | 2187.1 (1339.5, 3608) | 1424.2 (874.2, 1974.3) | 0.8182 | 7826.7 (1602.5, 27031.3) | 11264.7 (3694, 20389.9) | 5159.9 (832.9, 21719.3) | 0.2717 |
| C-peptide AUC | 583.4 (107.4, 981.4) | 533.9 (381.5, 754.5) | 531.4 (383, 679.8) | 0.8930 | 1084.2 (1084.2, 1084.2) | 1265.7 (650.5, 1655.4) | 865.9 (313.2, 1477.6) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[4 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0034_0332.gif) ![[3 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0033_0332.gif) ![[7 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0037_0332.gif)
|
| HOMA B | 20.1 (13.3, 43.8) | 15.4 (10.1, 45.4) | 8.9 (6.7, 11.2) | 0.6622 | 58.3 (19.2, 202.8) | 104.3 (37.4, 270.9) | 44.7 (6.7, 194.8) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[3 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0033_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[5 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0035_0332.gif)
|
| HOMA IR | 0.3 (0.1, 0.6) | 0.3 (0.2, 0.5) | 0.5 (0.1, 0.8) | 1.0000 | 0.8 (0.3, 3) | 2.7 (1, 5.4) | 3.3 (0.4, 10.2) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[4 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0034_0332.gif) ![[8 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0038_0332.gif) ![[6 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0036_0332.gif)
|
| QUICKI | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.5) | 0.5 (0.4, 0.6) | 1.0000 | 0.4 (0.3, 0.5) | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.4) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[3 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0033_0332.gif) ![[4 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0034_0332.gif) ![[6 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0036_0332.gif)
|
| MATSUDA | 23.6 (9.1, 31.4) | 19 (11.4, 31.1) | 26.9 (26.9, 26.9) | 0.0854 | 5.6 (1.8, 22.9) | 2.9 (1.3, 7.8) | 2.9 (0.9, 20.1) | 0.2205 |
| Insulinogenic index | 0.3 (0.2, 1) | 0.3 (-0.7, 0.6) | 0.2 (0.1, 0.3) | 0.5316 | 1.2 (0.2, 5.1) | 1.3 (0.4, 1.9) | 0.2 (0.1, 1.8) |
![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) . .![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0030_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0032_0332.gif) ![[4 with combining low line]](https://www.rsc.org/images/entities/b_i_char_0034_0332.gif)
|
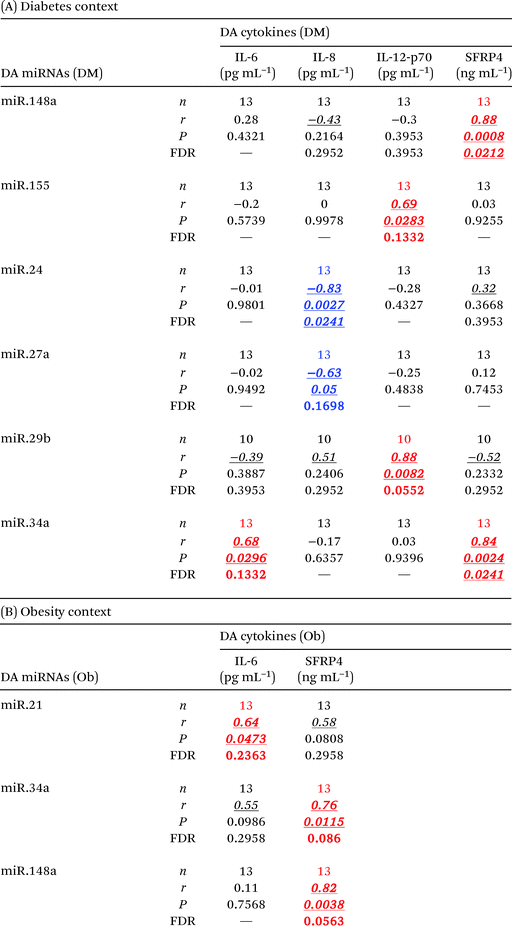
Cytokines in the diabetes context
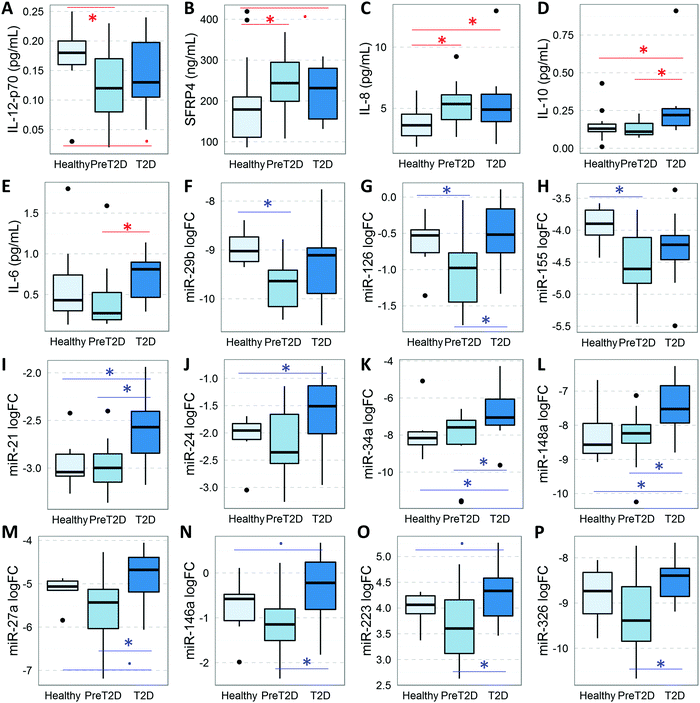
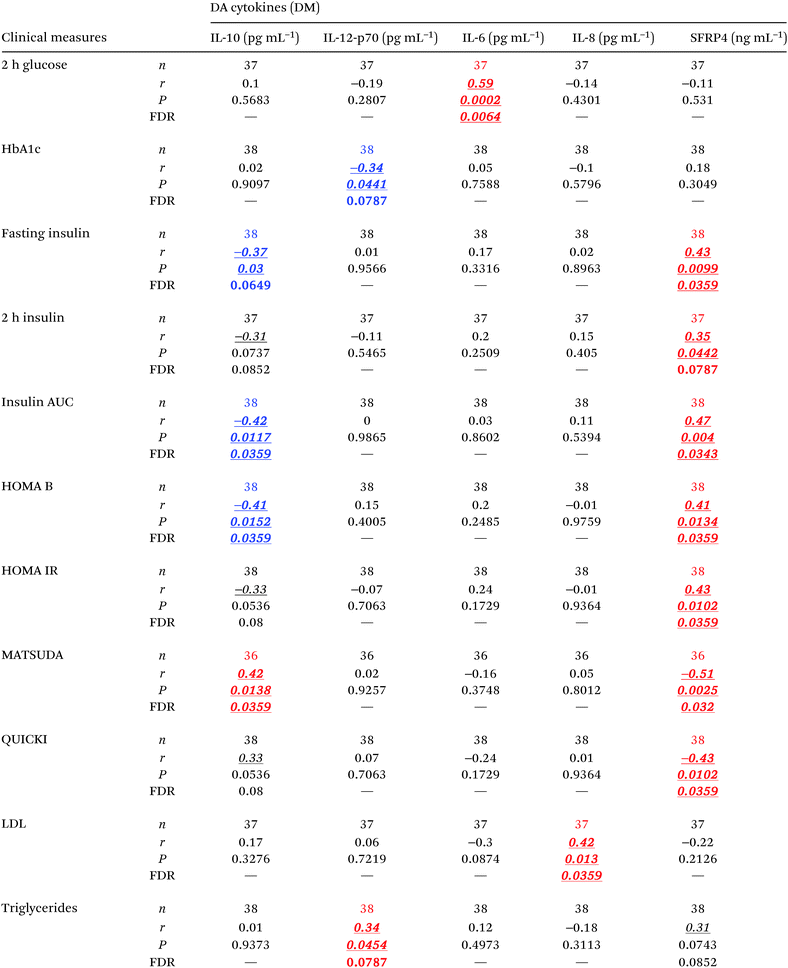
To assess the relative levels of circulating cytokines (this Section) and miRNAs (following Section) during diabetes development, we measured their respective serum levels in subjects at two distinct stages of the disease and compared them to healthy controls by implementing mathematical models that accounted for the confounding effects of age, gender, and BMI. Serum levels of IL-6, IL-8, IL-10, IL-12 (p70), and SFRP4 changed significantly (P < 0.05, FDR < 0.13) in subjects with prediabetes and/or T2D (Fig. 1A–E and Table 2A–C). Specifically, the levels of pro-inflammatory IL-8 were significantly increased in subjects with prediabetes, as compared to healthy controls and remained significantly elevated in T2D subjects, while anti-inflammatory IL-10 was significantly increased only in T2D. The levels of pro-inflammatory IL-12 (p70) were significantly reduced in subjects with prediabetes and tended to remain low in T2D (P = 0.078, FDR = 0.117). The level of pro-inflammatory/anti-angiogenic SFRP4 was significantly increased in subjects with prediabetes as compared to healthy controls, and tended to remain increased in T2D (P = 0.088, FDR = 0.132). IL-6, on the other hand, appeared to be significantly increased in the T2D group as compared to the prediabetes group. In addition, partial correlation analysis (adjusting for age, gender, and BMI) revealed significant associations (r > 0.3, P < 0.05, FDR < 0.08) of these cytokines with important clinical parameters related to glycemic control, β cell function, and systemic insulin sensitivity/resistance (Table 3). In particular, the levels of IL-12 inversely correlated with HbA1c levels, whereas the levels of SFRP-4 and IL-10 co-associated with fasting insulin and insulin AUC, as well as with the HOMA B and MATSUDA indices. SFRP4 also significantly and positively correlated with the insulin resistance index HOMA IR and negatively with the QUICKI index. On the other hand, IL-8 and IL-12 were significantly associated with cardiometabolic risk factors LDL and triglycerides.| Cytokine comparisons | |||
|---|---|---|---|
| A | |||
| PreT2D–healthy | Difference | P value | FDR |
| SFRP4 (ng mL−1) | 51.34 | 0.042 | 0.126 |
| IL-8 (pg mL−1) | 1.62 | 0.014 | 0.042 |
| IL-12.p70 (pg mL−1) | −0.05 | 0.011 | 0.033 |
| B | |||
|---|---|---|---|
| T2D–healthy | Difference | P value | FDR |
| IL-8 (pg mL−1) | 1.77 | 0.035 | 0.053 |
| IL-10 (pg mL−1) | 0.13 | 0.030 | 0.045 |
| C | |||
|---|---|---|---|
| T2D–PreT2D | Difference | P value | FDR |
| IL-6 (pg mL−1) | 0.27 | 0.023 | 0.069 |
| IL-10 (pg mL−1) | 0.15 | 0.007 | 0.021 |
| D | |||
|---|---|---|---|
| Obesity–lean | Difference | P value | FDR |
| SFRP4 (ng mL−1) | 89.41 | 0.005 | 0.018 |
| TNFα (pg mL−1) | 0.30 | 0.001 | 0.006 |
| IFNγ (pg mL−1) | 1.49 | 0.048 | 0.123 |
| IL-6 (pg mL−1) | 0.45 | 0.001 | 0.006 |
| MicroRNA comparisons | |||
|---|---|---|---|
| E | |||
| PreT2D–healthy | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FC FC |
P value | FDR |
| miR-29b | −0.75 | 0.041 | 0.123 |
| miR-126 | −0.38 | 0.032 | 0.048 |
| miR-155 | −0.58 | 0.013 | 0.039 |
| F | |||
|---|---|---|---|
| T2D–healthy | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FC FC |
P value | FDR |
| miR-21 | 0.32 | 0.014 | 0.021 |
| miR-24 | 0.49 | 0.026 | 0.039 |
| miR-34a | 1.03 | 0.011 | 0.030 |
| miR-148a | 0.84 | 0.006 | 0.009 |
| G | |||
|---|---|---|---|
| T2D–PreT2D | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FC FC |
P value | FDR |
| miR-21 | 0.36 | 0.003 | 0.009 |
| miR-24 | 0.61 | 0.013 | 0.039 |
| miR-27a | 0.71 | 0.010 | 0.030 |
| miR-34a | 1.47 | 0.020 | 0.030 |
| miR-126 | 0.52 | 0.007 | 0.021 |
| miR-146a | 0.74 | 0.014 | 0.042 |
| miR-148a | 0.91 | 0.006 | 0.009 |
| miR-223 | 0.64 | 0.009 | 0.027 |
| miR-326 | 0.79 | 0.014 | 0.042 |
| H | |||
|---|---|---|---|
| Obesity–lean | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FC FC |
P value | FDR |
| miR-21 | 0.33 | 0.004 | 0.024 |
| miR-24 | 0.74 | 0.002 | 0.024 |
| miR-25 | −0.44 | 0.039 | 0.085 |
| miR-27a | 0.60 | 0.018 | 0.054 |
| miR-34a | 1.18 | 0.031 | 0.083 |
| miR-93 | −0.48 | <0.001 | <0.001 |
| miR-126 | 0.32 | 0.035 | 0.084 |
| miR-146a | 0.73 | 0.005 | 0.024 |
| miR-148a | 0.87 | 0.005 | 0.024 |
| miR-150 | −0.67 | 0.016 | 0.054 |
| miR-152 | 0.66 | 0.049 | 0.096 |
| miR-223 | 0.58 | 0.006 | 0.024 |
miRNAs in the diabetes context
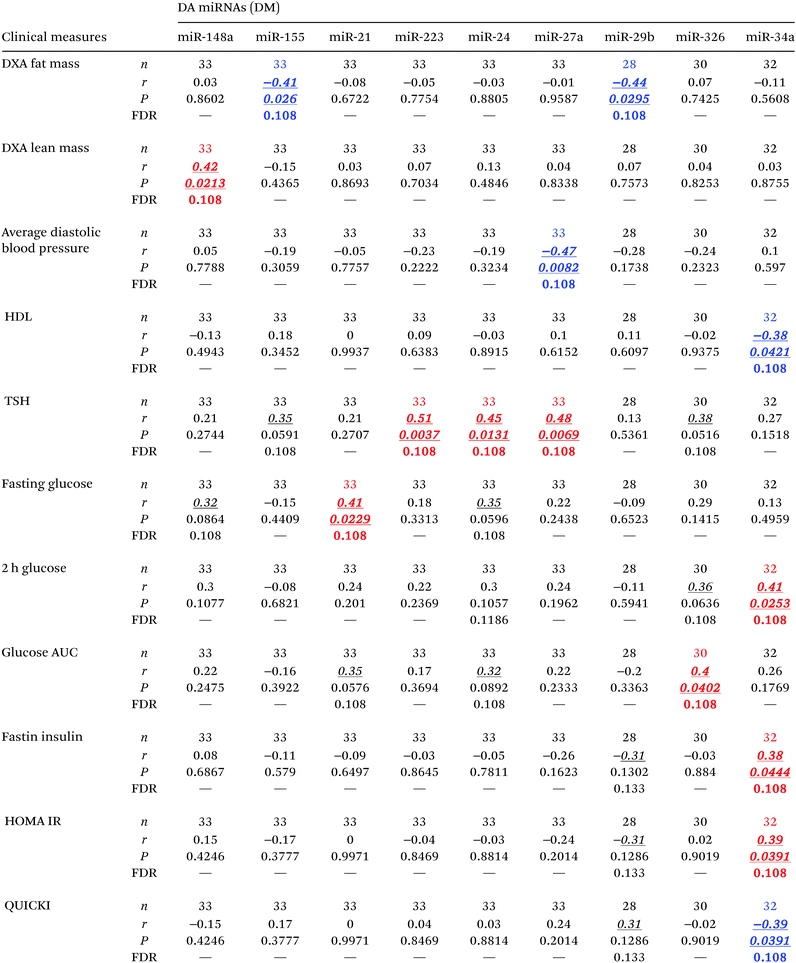
To assess the relative abundance of miRNAs in the circulation, we measured a panel of miRNAs previously reported to change in subjects with diabetes,10 and modeled their levels as described in the Material and methods section, adjusting for age, gender, and BMI. As shown in Fig. 1F–P and Table 2E–G, three miRNAs (i.e., miR-29b, miR-126, and miR-155) were significantly reduced (P < 0.05, FDR < 0.13) in the circulation of subjects with prediabetes. On the other hand, miR-21, miR-24.1, miR-34a, and miR-148a were significantly elevated (P < 0.05, FDR < 0.05) in the circulation of subjects with T2D. Four other miRNAs (i.e., miR-27a, miR-146a, miR-223, and miR-326) also displayed significantly elevated levels in the T2D group as compared to the prediabetes group. In addition, we found that the differentially abundant circulating miRNAs correlate with measures of glucose metabolism and β cell function including fasting glucose, 2 h glucose, glucose AUC, fasting insulin, HOMA IR, and QUICKI (Table 4).Cytokines in the obesity context
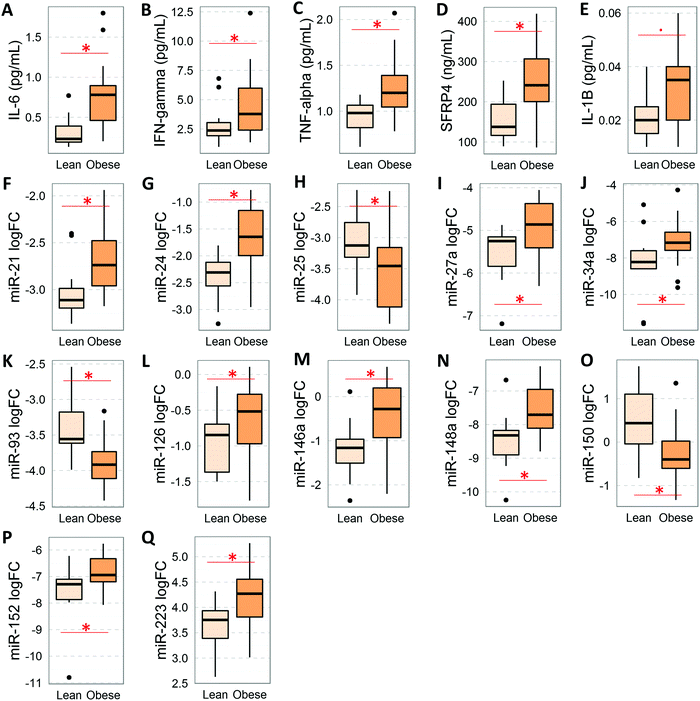
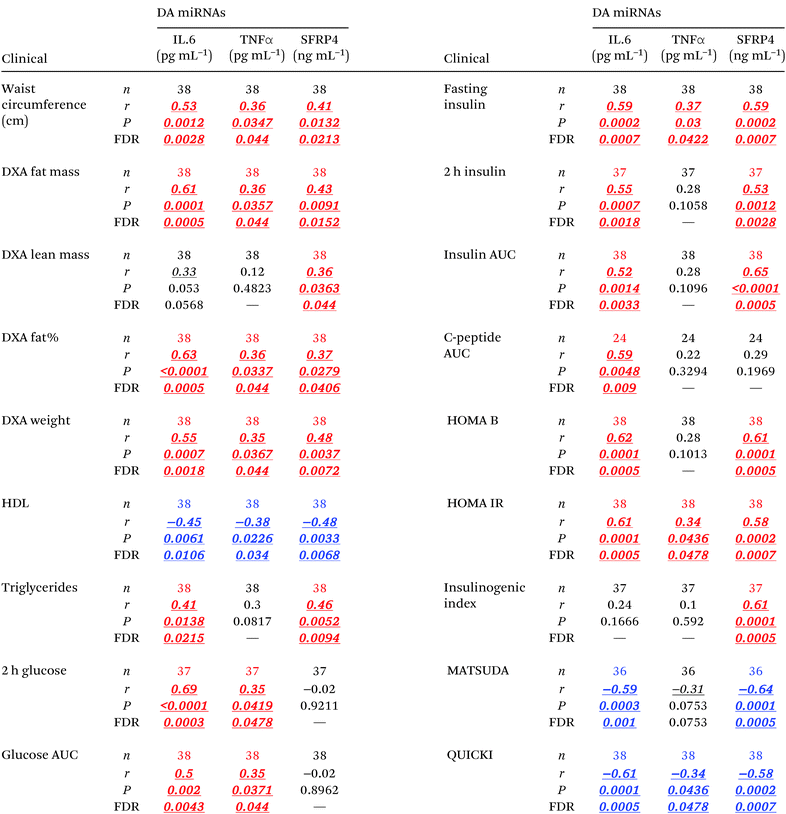
To assess the effect of obesity on the levels of circulating cytokines (this section) and miRNAs (following section), we implemented an alternative mathematical model that accounted for the confounding effects of age, gender, and diabetes status. We found that the serum levels of IL-6, IFNγ, TNFα, and SFRP4 were significantly elevated in people with obesity (Fig. 2A–D and Table 2D). In addition, cytokine IL-1β also displayed a strong trend (P = 0.056) towards elevation in the circulation levels in people with obesity (Fig. 2E). Remarkably, these cytokines displayed a large number of statistically significant correlations (r > 0.3, P < 0.05, FDR < 0.05) with body composition measures and measures of cardiometabolic risk factors, β cell function, glucose-stimulated insulin release, and systemic insulin sensitivity/resistance (Table 5). In particular, significant correlations (r > 0.6, P < 0.0005, FDR < 0.001) were detected for SFRP4 and IL-6 with insulin baseline levels, 2 hour insulin levels, insulin AUC, HOMA B, HOMA IR, QUICKI, and the insulinogenic index among others.miRNAs in the obesity context
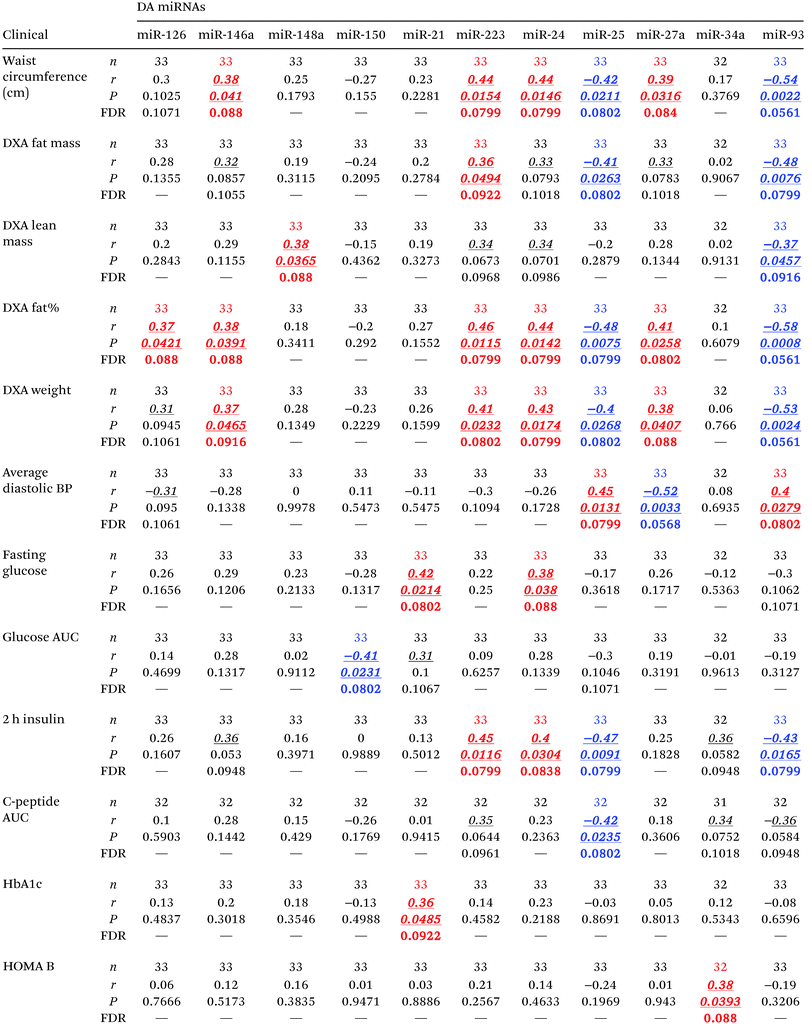
As shown in Fig. 2F–Q and Table 2H, the levels of 12 serum miRNAs (i.e., miR-21, miR-24.1, miR-27a, miR-34a, miR-126, miR-146a, miR-148a, miR-152, and miR-223 with elevated levels, and miR-25, miR-93, and miR-150 with reduced levels in the obese group) were affected by the obesity status of the subjects, independently of the diabetes stage. These miRNAs appeared to be directly involved with the development and/or progression of obesity. Notably, increased miR-152 was reported to indirectly upregulate SFRP4 expression.33 Consistent with this, SFRP4 was also elevated in overweight/obese subjects. Interestingly, several of these miRNAs (i.e., miR-21, miR-24.1, miR-27a, miR-34a, miR-126, miR-146a, miR-148a, and miR-223) were concomitantly associated with diabetes independent of obesity and BMI status. In addition, we found that several of these differentially abundant circulating miRNAs displayed significant correlations with measures of glycemic control and β cell function including HbA1c, fasting glucose, glucose AUC, 2 h insulin, C-peptide AUC, and HOMA B, as well as with body composition measures such as fat mass and percentage, lean mass, total weight, and waist circumference (Table 6).Cytokine-miRNA correlations in the context of either diabetes or obesity
Interestingly, only a few but strong correlations (r > 0.6) were found to be statistically significant (P < 0.05, FDR < 0.24) between differentially abundant circulating cytokines and differentially abundant circulating miRNAs in the context of either diabetes or obesity (Table 7). These cytokine–miRNA correlations were all, with the exception of two cases, of positive sign. This suggested that the associations represent mostly indirect interactions between these signaling/regulatory molecules. However, the levels of circulating IL-8 were strongly anti-correlated with the levels of circulating miR-24.1 (r = −0.83, P = 0.0027, FDR = 0.0241) and miR-27a (r = −0.63, P = 0.05, FDR = 0.17). These particular negative correlations indicate that miR-24.1 and miR-27a may directly target and downregulate IL-8 production and/or secretion in specific tissues. Notably, miR-34a consistently correlated with SFRP4 levels in the context of both obesity and diabetes.Discussion
Cytokine profiles detected in our study cohort are consistent with the activation of an inflammatory response during the early stages of diabetes development, driven by deregulation of specific pro-inflammatory cytokines (i.e., IL-6, IL-8, IL-12, and SFRP4). The early changes in the levels of IL8, IL-12, and SFRP4 in the diabetic context have potential as early biomarkers of disease development. The elevation of anti-inflammatory IL-10 levels in the T2D stage suggests an adaptive response of the organism in an effort to cope with increasing inflammatory signaling. In addition, IL-10 and IL-12 are key cytokines that drive the balance between Th1 and Th2 effector (Teff) T helper cells.34 Interleukin IL-10 suppresses Th1 cells, which require IL-12 for differentiation.35 Therefore, the reduced levels of IL-12 in the prediabetes stage and the elevated IL-10 in the T2D stage suggest a continuous organismal effort for halting the activation of the Th1 subpopulation of Teff cells (main producers of pro-inflammatory INFγ), possibly as a mechanism to maintain homeostasis in relevant tissues. Notably, several studies have reported the augmentation of the Th1 cell subset and the causal involvement of this phenomenon in inflammation and insulin resistance in mouse models of diabetes, and in T1D and T2D in humans.35 The significantly elevated level of IL-8 in subjects with prediabetes and T2D suggests that this cytokine is an important driver of inflammation in subjects at high risk of developing diabetes and progressing through diabetes. Supporting our reasoning, IL-8 has been reported as significantly upregulated in T2D patients36 and is the most consistently upregulated cytokine in both adults and children with T1D.37 As reported previously, the production of IL-8 could be induced by TNFα and IL-6.38 The elevation of IL-6 in the T2D group could therefore explain, at least in part, the increase in circulating IL-8. In addition, IL-8 has a key role as a neutrophil chemoattractant and activator, and is a primary cytokine secreted by neutrophils themselves and M1 inflammatory macrophages.39 Consequently, our results suggest that an inflammatory process involving neutrophil and macrophage activation may be related to the development of T2D. Indeed, it is widely acknowledged that proinflammatory macrophages in adipose tissues are the primary cell type responsible for the inflammation in diabetes,40 however, the diabetogenic role of neutrophils has only recently started to gain support.41On the other hand, all the cytokines affected by obesity and diabetes in our cohort (i.e., IL-8, IL-6, IL-10, IL-12, IFNγ, TNFα, and SFRP4) have known functions in angiogenesis,42–44 which plays a known key role during the development of obesity. Because impaired adipose tissue angiogenesis in obesity is a contributing factor to insulin resistance and metabolic disease,45 which are early steps in the development of T2D, our data suggest that this particular circulating cytokine profile might play a causative role in the development of T2D. Therefore, modulation of these cytokines, individually or simultaneously, may show therapeutic effects. Remarkably, blockage of the IL-8 receptor with reparixin enhances pancreatic islet survival after transplantation in humans and reverts T1D in mice46 implying that reparixin may also prove useful in the treatment of T2D. Importantly, our results corroborate an emerging role of SFRP4 in obesity47 and implicate its deregulation in the early development of T2D. Other studies have implicated SFRP4 elevation in human T2D.48 Previously, we demonstrated that circulating levels of SFRP4 were elevated in a different cohort of individuals with obesity, that abdominal adipose tissues are major contributors for circulating SFRP4, and that SFRP4 has an important role in adipose tissue pathophysiology in obesity.47 The strong and large number of correlations detected between SFRP4 and multiple measures of glycemic control and insulin action in this study cohort, both dependently and independently of the obesity status (Tables 2 and 4), underscore the role recently suggested for SFRP4 in the development and progression of diabetes.
Similarly, the levels of specific miRNAs are altered in the circulation of subjects with prediabetes and T2D, independently of the BMI (which defines our selection of obesity status) of the subjects, as well as in subjects with obesity, independently of the diabetes status. The role of circulating miRNAs in the pathogenesis of diabetes and their potential use as biomarkers of disease development, progression, and response to treatment has been reported.49–51 However, little is known about their interplay/crosstalk with immune mediators as such. In this regard, we detected mostly positive correlations among the differentially abundant circulating miRNAs and cytokines (Table 7). The positive associations suggest that these miRNAs are not directly targeting the respective cytokines, but are indirectly interacting. Supporting this reasoning, Wang et al. found that TGF-β, a key cytokine involved in the pathogenesis of many diseases,52 stimulates the secretion of miR-130b from adipocytes (but not from myocytes) and that circulating miR-130b can enter the muscle cells and reduce the expression of genes such as PGC-1α, which plays a key role in lipid oxidation in muscle tissues.52 In another example of indirect association, Xiang et al. showed that one of the mechanisms by which chronic inflammation contributes to cancer involves the IL-6-dependent activation of the transcription factor STAT3, which consequently activates the promoter of miR-146b.53 The upregulation of miR-146b, in turn, inhibits the nuclear factor κB-dependent production of IL-6, thereby creating a feedback loop.53 Other authors reported that IL-21 inhibits the production of chemokines that favor the crosstalk of CD40-activated chronic lymphocytic leukemia cells with supportive cells within the microenvironment through a miRNA-mediated mechanism involving miR-663b.54 Alternatively, the positive correlation might be due to miRNAs being released as an adaptive response to counter the effects of cytokine-induced inflammation. Relevantly, Nesca et al. reported that “obesity and insulin resistance trigger adaptations in the levels of particular miRNAs to allow sustained β cell function, and that additional miRNA deregulation negatively impacting on insulin-secreting cells may cause β cell demise and diabetes manifestation”.55 Similar miRNA-driven adaptive/homeostatic phenomena have been described during early stages of distinct disorders such as alcohol addiction.56 The two significant negative associations detected between the levels of miR-24.1 and IL-8 and between miR-27a and IL-8 in our study suggest that these miRNAs could directly target the transcript of the IL8 gene. Indeed, the IL8 transcript is predicted using software RNAhybrid57 and miRWalk58 to harbor miR-24.1 and miR-27a binding sites in its 3′-UTR. Although cytokines are not commonly reported as miRNA targets, several instances of validated interactions have been documented, such as the targeting of IL-8 by miR-9321 and miR-520b,22 among others cataloged by miRWalk.
Although these results appear interesting from our viewpoint, several limitations of our study need to be noted. Because the cohorts analyzed in this study were small and the study was cross-sectional by design, these findings must be validated in separate and larger cohorts followed up longitudinally to correlate these findings with the natural progression of diabetes and be able to make causal inferences. The study warrants further characterization of miRNAs and cytokines in specific potential source or target tissues such as peripheral blood mononuclear cells and adipose, muscle, and vascular tissues, among others.
Conclusions
Our study demonstrates a potential role for circulating cytokines and miRNAs in contributing to the pathophysiological changes in subjects with prediabetes and T2D and pinpointed the early biomarker potential of cytokines (i.e., IL-8, IL-12, and SFRP4) and miRNAs (i.e., miR-29b, miR-126, and miR-155) as markers of prediabetes. By early detection of an increased risk for development of the disease, we could more effectively implement preventive measures aimed at halting the diabetes epidemic. Our results suggest that the unbalanced pro-/anti-inflammatory/angiogenic cytokine levels and the deregulation of immune and metabolically-involved miRNAs contribute to the remodeling of a diabetogenic molecular network that eventually leads to disease development.Duality of interest
This study was funded by startup funds granted to A. A. S. by Florida Hospital. No other potential conflicts of interest relevant to this article were reported.Author contributions
Y. O. N. L. conducted experiments, analyzed data, and wrote the manuscript and G. G. conducted experiments, analyzed data, and contributed to manuscript writing. Correspondences regarding the data analysis and “R package” should be addressed to Y. O. N. L. (Yury.Nunez-Lopez@flhosp.org). A. A. S. designed the study, analyzed the data, and contributed to manuscript writing, editing, and critical revision of the manuscript, and authorized the manuscript.Acknowledgements
We would like to thank Florida Hospital for making the funds available to A. A. S. to conduct this study.References
- C. Herder, M. Carstensen and D. M. Ouwens, Anti-inflammatory cytokines and risk of type 2 diabetes, Diabetes, Obes. Metab., 2013, 15(suppl 3), 39–50 Search PubMed.
- O. Osborn and J. M. Olefsky, The cellular and signaling networks linking the immune system and metabolism in disease, Nat. Med., 2012, 18(3), 363–374 Search PubMed.
- H. K. Akerblom, M. Knip, H. Hyoty, H. Reijonen, S. Virtanen and E. Savilahti, et al., Interaction of genetic and environmental factors in the pathogenesis of insulin-dependent diabetes mellitus, Clin. Chim. Acta, 1997, 257(2), 143–156 Search PubMed.
- B. Vandanmagsar, Y. H. Youm, A. Ravussin, J. E. Galgani, K. Stadler and R. L. Mynatt, et al., The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance, Nat. Med., 2011, 17(2), 179–188 Search PubMed.
- D. McGonagle and M. F. McDermott, A proposed classification of the immunological diseases, PLoS Med., 2006, 3(8), e297 Search PubMed.
- D. Bartel, MicroRNAs: genomics, biogenesis, mechanism, and function, Cell, 2004, 116, 281–297 Search PubMed.
- H. Guo, N. T. Ingolia, J. S. Weissman and D. P. Bartel, Mammalian microRNAs predominantly act to decrease target mRNA levels, Nature, 2010, 466(7308), 835–840 Search PubMed.
- M. R. Wing, A. Ramezani, H. S. Gill, J. M. Devaney and D. S. Raj, Epigenetics of progression of chronic kidney disease: fact or fantasy?, Semin. Nephrol., 2013, 33(4), 363–374 CrossRef CAS PubMed.
- R. C. Friedman, K. K. Farh, C. B. Burge and D. P. Bartel, Most mammalian mRNAs are conserved targets of microRNAs, Genome Res., 2009, 19(1), 92–105 Search PubMed.
- A. A. Seyhan, microRNAs with different functions and roles in disease development and as potential biomarkers of diabetes: progress and challenges, Mol. BioSyst., 2015, 11(5), 1217–1234 Search PubMed.
- M. Bonafe and F. Olivieri, Circulating microRNAs in aging, Oncotarget, 2015, 6(3), 1340–1341 Search PubMed.
- T. J. Kirby and J. J. McCarthy, MicroRNAs in skeletal muscle biology and exercise adaptation, Free Radical Biol. Med., 2013, 64, 95–105 Search PubMed.
- Y. O. Nunez and R. D. Mayfield, Understanding Alcoholism Through microRNA Signatures in Brains of Human Alcoholics, Front. Genet., 2012, 3, 43 CAS.
- B. Victoria, J. M. Dhahbi, Y. O. Nunez Lopez, L. Spinel, H. Atamna and S. R. Spindler, et al., Circulating microRNA signature of genotype-by-age interactions in the long-lived Ames dwarf mouse, Aging Cell, 2015, 14(6), 1055–1066 Search PubMed.
- W. C. Cho, Circulating MicroRNAs as Minimally Invasive Biomarkers for Cancer Theragnosis and Prognosis, Front. Genet., 2011, 2, 7 Search PubMed.
- F. J. Ortega, J. M. Mercader, J. M. Moreno-Navarrete, O. Rovira, E. Guerra and E. Esteve, et al., Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization, Diabetes Care, 2014, 37(5), 1375–1383 Search PubMed.
- J. Raffort, C. Hinault, O. Dumortier and E. Van Obberghen, Circulating microRNAs and diabetes: potential applications in medical practice, Diabetologia, 2015, 58(9), 1978–1992 Search PubMed.
- H. Schwarzenbach, N. Nishida, G. A. Calin and K. Pantel, Clinical relevance of circulating cell-free microRNAs in cancer, Nat. Rev. Clin. Oncol., 2014, 11(3), 145–156 CrossRef CAS PubMed.
- B. Victoria Martinez, J. M. Dhahbi, Y. O. Nunez Lopez, K. Lamperska, P. Golusinski and L. Luczewski, et al., Circulating small non coding RNA signature in head and neck squamous cell carcinoma, Oncotarget, 2015, 6(22), 19246–19263 Search PubMed.
- R. Zhou, S. P. O'Hara and X. M. Chen, MicroRNA regulation of innate immune responses in epithelial cells, Cell. Mol. Immunol., 2011, 8(5), 371–379 Search PubMed.
- E. Fabbri, M. Borgatti, G. Montagner, N. Bianchi, A. Finotti and I. Lampronti, et al., Expression of microRNA-93 and Interleukin-8 during Pseudomonas aeruginosa-mediated induction of proinflammatory responses, Am. J. Respir. Cell Mol. Biol., 2014, 50(6), 1144–1155 CrossRef PubMed.
- N. Hu, J. Zhang, W. Cui, G. Kong, S. Zhang and L. Yue, et al., miR-520b regulates migration of breast cancer cells by targeting hepatitis B X-interacting protein and interleukin-8, J. Biol. Chem., 2011, 286(15), 13714–13722 Search PubMed.
- M. Baou, J. D. Norton and J. J. Murphy, AU-rich RNA binding proteins in hematopoiesis and leukemogenesis, Blood, 2011, 118(22), 5732–5740 Search PubMed.
- D. Barbagallo, S. Piro, A. G. Condorelli, L. G. Mascali, F. Urbano and N. Parrinello, et al., miR-296-3p, miR-298-5p and their downstream networks are causally involved in the higher resistance of mammalian pancreatic alpha cells to cytokine-induced apoptosis as compared to beta cells, BMC Genomics, 2013, 14, 62 Search PubMed.
- N. Wang, H. Liang and K. Zen, Molecular mechanisms that influence the macrophage m1–m2 polarization balance, Front. Immunol., 2014, 5, 614 Search PubMed.
- American Diabetes A., Standards of medical care in diabetes, Diabetes Care, 2014, 37(suppl 1), S14–S80 Search PubMed.
- E. S. Edgington, P. Onghena, Randomization Tests: Chapman and Hall/CRC, 2007, p. 376 Search PubMed.
- R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2016 Search PubMed.
- T. Hothorn, K. Hornik and M. A. van de Wiel, A. Zeileis Implementing a Class of Permutation Tests: The coin Package, J. Stat. Software, 2008, 1(8), 1–23 Search PubMed.
- H. Strasser and C. Weber, On the asymptotic theory of permutation statistics, Vienna University of Economics and Business Administration, 1999 Search PubMed.
- S. Kim, ppcor: An R Package for a Fast Calculation to Semi-partial Correlation Coefficients, Commun. Stat. Appl. Methods, 2015, 22(6), 665–674 Search PubMed.
- Y. Benjamini and Y. Hochberg, Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing, J. R. Stat. Soc. Series B: Stat. Methodol., 1995, 57(1), 289–300 Search PubMed.
- C. G. Miao, Y. Y. Yang, X. He, C. Huang, Y. Huang and D. Qin, et al., MicroRNA-152 modulates the canonical Wnt pathway activation by targeting DNA methyltransferase 1 in arthritic rat model, Biochimie, 2014, 106, 149–156 Search PubMed.
- H. Yang, Y. H. Youm, B. Vandanmagsar, A. Ravussin, J. M. Gimble and F. Greenway, et al., Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance, J. Immunol., 2010, 185(3), 1836–1845 CrossRef CAS PubMed.
- M. Kornete, E. S. Mason and C. A. Piccirillo, Immune Regulation in T1D and T2D: Prospective Role of Foxp3+ Treg Cells in Disease Pathogenesis and Treatment, Front. Endocrinol., 2013, 4, 76 Search PubMed.
- C. Herder, J. Baumert, B. Thorand, S. Martin, H. Lowel and H. Kolb, et al., Chemokines and incident coronary heart disease: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002, Arterioscler., Thromb., Vasc. Biol., 2006, 26(9), 2147–2152 Search PubMed.
- A. B. Erbağci, M. Tarakioğlu, Y. Cokun, E. Sivasli and E. Sibel Namiduru, Mediators of inflammation in children with type I diabetes mellitus: cytokines in type I diabetic children, Clin. Biochem., 2001, 34(8), 645–650 Search PubMed.
- M. Baggiolini, B. Dewald and B. Moser, Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines, Adv. Immunol., 1994, 55, 97–179 Search PubMed.
- M. M. Mohamed, E. A. El-Ghonaimy, M. A. Nouh, R. J. Schneider, B. F. Sloane and M. El-Shinawi, Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties, Int. J. Biochem. Cell Biol., 2014, 46, 138–147 Search PubMed.
- A. M. Johnson and J. M. Olefsky, The origins and drivers of insulin resistance, Cell, 2013, 152(4), 673–684 Search PubMed.
- X. Rao, J. Zhong and Q. Sun, The heterogenic properties of monocytes/macrophages and neutrophils in inflammatory response in diabetes, Life Sci., 2014, 116(2), 59–66 Search PubMed.
- G. Neufeld and O. Kessler, Pro-angiogenic cytokines and their role in tumor angiogenesis, Cancer Metastasis Rev., 2006, 25(3), 373–385 Search PubMed.
- D. Ribatti and E. Crivellato, Immune cells and angiogenesis, J. Cell. Mol. Med., 2009, 13(9A), 2822–2833 Search PubMed.
- A. Muley, S. Majumder, G. K. Kolluru, S. Parkinson, H. Viola and L. Hool, et al., Secreted frizzled-related protein 4: an angiogenesis inhibitor, Am. J. Pathol., 2010, 176(3), 1505–1516 Search PubMed.
- S. Corvera and O. Gealekman, Adipose tissue angiogenesis: impact on obesity and type-2 diabetes, Biochim. Biophys. Acta, 2014, 1842(3), 463–472 Search PubMed.
- A. Citro, A. Valle, E. Cantarelli, A. Mercalli, S. Pellegrini and D. Liberati, et al., CXCR1/2 Inhibition Blocks and Reverts Type 1 Diabetes in Mice, Diabetes, 2015, 64(4), 1329–1340 Search PubMed.
- G. Garufi, A. A. Seyhan and M. Pasarica, Elevated secreted frizzled-related protein 4 in obesity: a potential role in adipose tissue dysfunction, Obesity, 2015, 23(1), 24–27 CrossRef CAS PubMed.
- T. Mahdi, S. Hanzelmann, A. Salehi, S. J. Muhammed, T. M. Reinbothe and Y. Tang, et al., Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes, Cell Metab., 2012, 16(5), 625–633 Search PubMed.
- C. Guay, E. Roggli, V. Nesca, C. Jacovetti and R. Regazzi, Diabetes mellitus, a microRNA-related disease?, Transl. Res., 2011, 157(4), 253–264 Search PubMed.
- A. A. Seyhan, microRNAs with different functions and roles in disease development and as potential biomarkers of diabetes: progress and challenges, Mol. BioSyst., 2015, 11(5), 1217–1234 Search PubMed.
- A. A. Seyhan, Y. O. Nunez Lopez, H. Xie, F. Yi, C. Mathews and M. Pasarica, et al., Pancreas-enriched miRNAs are altered in the circulation of subjects with diabetes: a pilot cross-sectional study, Sci. Rep., 2016, 6, 31479 Search PubMed.
- N. Zamani and C. W. Brown, Emerging roles for the transforming growth factor-beta superfamily in regulating adiposity and energy expenditure, Endocr. Rev., 2011, 32(3), 387–403 Search PubMed.
- M. Xiang, N. J. Birkbak, V. Vafaizadeh, S. R. Walker, J. E. Yeh and S. Liu, et al., STAT3 induction of miR-146b forms a feedback loop to inhibit the NF-kappaB to IL-6 signaling axis and STAT3-driven cancer phenotypes, Sci. Signaling, 2014, 7(310), ra11 Search PubMed.
- L. De Cecco, M. Capaia, S. Zupo, G. Cutrona, S. Matis and A. Brizzolara, et al., Interleukin 21 Controls mRNA and MicroRNA Expression in CD40-Activated Chronic Lymphocytic Leukemia Cells, PLoS One, 2015, 10(8), e0134706 Search PubMed.
- V. Nesca, C. Guay, C. Jacovetti, V. Menoud, M. L. Peyot and D. R. Laybutt, et al., Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes, Diabetologia, 2013, 56(10), 2203–2212 Search PubMed.
- Y. O. Nunez, J. M. Truitt, G. Gorini, O. N. Ponomareva, Y. A. Blednov and R. A. Harris, et al., Positively correlated miRNA-mRNA regulatory networks in mouse frontal cortex during early stages of alcohol dependence, BMC Genomics, 2013, 14, 725 Search PubMed.
- J. Krüger and M. Rehmsmeier, RNAhybrid: microRNA target prediction easy, fast and flexible, Nucleic Acids Res., 2006, 34(suppl 2), W451–W454 Search PubMed.
- H. Dweep, C. Sticht, P. Pandey and N. Gretz, miRWalk–database: prediction of possible miRNA binding sites by “walking” the genes of three genomes, J. Biomed. Inf., 2011, 44(5), 839–847 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |