Persistent drought monitoring using a microfluidic-printed electro-mechanical sensor of stomata in planta†
Abstract
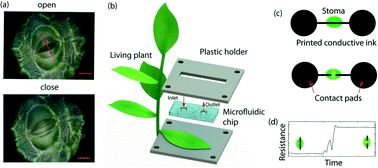
Stomatal function can be used effectively to monitor plant hydraulics, photosensitivity, and gas exchange. Current approaches to measure single stomatal aperture, such as mold casting or fluorometric techniques, do not allow real time or persistent monitoring of the stomatal function over timescales relevant for long term plant physiological processes, including vegetative growth and abiotic stress. Herein, we utilize a nanoparticle-based conducting ink that preserves stomatal function to print a highly stable, electrical conductometric sensor actuated by the stomata pore itself, repeatedly and reversibly for over 1 week. This stomatal electro-mechanical pore size sensor (SEMPSS) allows for real-time tracking of the latency of single stomatal opening and closing times in planta, which we show vary from 7.0 ± 0.5 to 25.0 ± 0.5 min for the former and from 53.0 ± 0.5 to 45.0 ± 0.5 min for the latter in Spathiphyllum wallisii. These values are shown to correlate with the soil water potential and the onset of the wilting response, in quantitative agreement with a dynamic mathematical model of stomatal function. A single stoma of Spathiphyllum wallisii is shown to distinguish between incident light intensities (up to 12 mW cm−2) with temporal latency slow as 7.0 ± 0.5 min. Over a seven day period, the latency in opening and closing times are stable throughout the plant diurnal cycle and increase gradually with the onset of drought. The monitoring of stomatal function over long term timescales at single stoma level will improve our understanding of plant physiological responses to environmental factors.


 Please wait while we load your content...
Please wait while we load your content...