Open Access Article
Open Access ArticleEsterification and hydrolysis of cellulose using oxalic acid dihydrate in a solvent-free reaction suitable for preparation of surface-functionalised cellulose nanocrystals with high yield†
D.
Li
 ,
J.
Henschen
,
J.
Henschen
 and
M.
Ek
and
M.
Ek
 *
*
KTH Royal Institute of Technology, School of Chemical Science and Engineering, Department of Fibre and Polymer Technology, Division of Wood Chemistry and Pulp Technology, Stockholm, Sweden. E-mail: monicaek@kth.se
First published on 13th November 2017
Abstract
A one-pot esterification and hydrolysis of cellulose was carried out by treating cellulose fibres with molten oxalic acid dihydrate. Each cellulose oxalate had a free carboxyl content above 1.2 mmol g−1 and an average molecular weight of approximately 40 kDa. Aqueous suspensions of the oxalates were sonicated to prepare cellulose nanocrystals with a gravimetric yield of 80.6%.
Cellulose nanomaterials or nanocellulose have attracted increased attention over the past 10 years due to the possibility of using these renewable nanoparticles to prepare materials with many interesting properties for several applications.1 Hitherto, the reported classic methods for preparing nanocellulose from plant fibres could generally be categorized into 2 groups: (1) preparation of cellulose nanocrystals (CNC) through strong mineral acid-catalysed hydrolysis followed by mechanical fibrillation,2–4 and (2) preparation of cellulose nanofibrils (CNF) through mechanical fibrillation of cellulose fibres in aqueous suspensions directly or following a pretreatment such as partial carboxymethylation, enzymatic treatment or 2,2,6,6-tetramethylpiperidine-1-oxy radical (TEMPO) mediated oxidation.5–10 Recently, new methods, such as using solutions of organic acids for the pretreatments of cellulose fibres to prepare cellulose nanomaterials with improved yields, surface functionalities, and/or thermal properties, have also been reported.11–14
Here, we report a simple and effective way of preparing functionalised cellulose nanocrystals with high yield. A one-pot esterification and hydrolysis of cellulose was carried out by mixing softwood dissolving pulp with molten oxalic acid dihydrate at 110 °C for 15 min, 30 min, 60 min, and 120 min, respectively, under constant mixing and reflux, to obtain cellulose oxalates (COX), denoted as COX15, COX30, COX60, COX120, respectively. Oxalic acid dihydrate is an inexpensive chemical and can industrially be produced from plant-based resources. It is a naturally abundant, biodegradable, non-volatile, and thus a sustainable reagent. Previously, oxalic acid dihydrate has been used as an esterification agent for wood fibres, whereas its dehydrate, oxalic acid, has been used in various solutions to hydrolyse pure cellulose for the preparation of cellulose nanocrystals.11,12,14 However, to the best of our knowledge, it has never been used in a water- or solvent-free system to convert pure cellulose fibres (dissolving pulp in this case) into cellulose derivatives.
In this study, oxalic acid dihydrate was used due to its relatively low melting point of 104–106 °C, which makes it possible to carry out a solvent-free reaction for the esterification of cellulose at 110 °C. The molten oxalic acid dihydrate could wet the fibres, taking part in simultaneous hydrolysis and derivatization of cellulose.
After the reaction, all samples were sufficiently washed by filtration and then Soxhlet extraction, to remove the excess oxalic acid dihydrate (see ESI†). After drying, cellulose oxalates were obtained as fine and white powders.
Fourier transform infrared spectra showed that all the cellulose oxalates exhibited broad absorption bands at 1739 cm−1 (Fig. S1, ESI†), corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carbonyl group.14 This signal indicates successful esterification of cellulose by oxalic acid dihydrate. However, it is difficult to differentiate the signals of carboxylic acids (–COOH) and ester bonds (–COO–) as they overlap. 13C NMR spectrum of COX15 was recorded to further study the structure of the cellulose oxalate. Two distinct peaks at 174 ppm and 157 ppm, corresponding to the carboxylic acid and the ester functional groups respectively, could be observed (Fig. S2, ESI†). This observation suggests not only the formation of covalent bonds as a result of the esterification of cellulose, but also the existence of carboxylic acid groups in the cellulose oxalate.
O stretching of the carbonyl group.14 This signal indicates successful esterification of cellulose by oxalic acid dihydrate. However, it is difficult to differentiate the signals of carboxylic acids (–COOH) and ester bonds (–COO–) as they overlap. 13C NMR spectrum of COX15 was recorded to further study the structure of the cellulose oxalate. Two distinct peaks at 174 ppm and 157 ppm, corresponding to the carboxylic acid and the ester functional groups respectively, could be observed (Fig. S2, ESI†). This observation suggests not only the formation of covalent bonds as a result of the esterification of cellulose, but also the existence of carboxylic acid groups in the cellulose oxalate.
To study the effect of the reaction time on the esterification, two titration-based determinations of carboxyl content were performed to quantify the total carboxyl content (TCC) and the free carboxyl content (FCC). Oxalic acid is a dicarboxylic acid where either one or both of the carboxyl groups can react with the hydroxyl groups of the cellulose. Therefore, the esterification of cellulose with oxalic acid dihydrate could lead to either crosslinking between two anhydrous glucose units or the introduction of free carboxylic acid functionalities in the cellulose. TCC is the total amount of both free carboxyl groups (–COOH) and bonded carboxyl groups/ester groups (–COO–) of the oxalates while FCC is just the number of free carboxyl groups. The ratio between FCC and TCC indicates the degree of crosslinking. For making nanocellulose, ideally, one carboxyl group of the oxalic acid shall bond to cellulose through an ester bond, while the other one should be left free. In this case, the ratio of FCC/TCC is 0.5, which indicates no crosslinking is present, while a ratio of 0 would indicate that all carboxyl groups take part in the formation of ester bonds with cellulose and there are no free carboxyl groups. The degree of substitution, total carboxyl content, free carboxyl content and the ratio of FCC/TCC of the different samples are shown in Table 1. After 15 min, the ratio of FCC/TCC was 0.47. As the reaction continued to 30 min, it slightly decreased to 0.43 and then became stable for over 2 h. This indicates that most of the oxalic acids bonded to cellulose through one of their two carboxyl groups, while the other one was left free. Both TCC and FCC increased the most during the first 30 min and reached a maximum after 60 min.
| Sample | Reaction time (min) | Gravimetric yield (%) | DS | TCC (mmol g−1) | FCC (mmol g−1) | Ratio of FCC/TCC |
|---|---|---|---|---|---|---|
| COX15 | 15 | — | 0.25 | 2.59 | 1.22 | 0.47 |
| COX30 | 30 | 99 | 0.34 | 3.15 | 1.34 | 0.43 |
| COX60 | 60 | 94 | 0.35 | 3.26 | 1.39 | 0.43 |
| COX120 | 120 | — | 0.33 | 3.07 | 1.30 | 0.42 |
The esterification of cellulose by oxalic acid dihydrate was a quick process as the DS reached 0.25 after only 15 min. Then, it increased slightly to 0.34 after another 15 min and then became stable. As it has been reported,15,16 approximately 5% of the total cellulose chains are located at less-ordered and accessible surfaces of fibrils or fibril aggregates. Considering the fact that the esterification of cellulose occurs most easily at the C6–OH (Fig. S3, ESI†), the theoretical DS of a cellulose ester prepared in a solvent free reaction without a pretreatment of cellulose (e.g., swelling) is 0.05. However, in the current study, all DS exceeded this value. It indicates that the esterification occurred not only on the accessible surfaces but also on the less-ordered but inaccessible surfaces (in this case, the theoretical DS can reach 0.25–0.5) between the fibrils within the fibril aggregates. It is proposed that oxalic acid dihydrate is able to reach and consequently functionalise parts of the inaccessible surfaces, due to swelling of the fibril aggregates with a low amount of water that was present in the reaction. Nevertheless, the strong intermolecular interactions among the cellulose chains hampered the further migration of oxalic acid towards the ordered and crystalline interior of the fibrils, so that the esterification could not proceed further and a maximum DS of 0.35 was obtained after a reaction time of 60 min.
The transformation of the fibres into very fine powders suggested a breakdown of the macromolecular structure, presumably arising from the hydrolysis of cellulose chains which was catalysed by oxalic acid. To verify this assumption, the molecular weight properties of the cellulose oxalates were determined by size exclusion chromatography.
The treatment of cellulose with oxalic acid dihydrate led to a substantial decrease in the molecular weight (Mw), as shown by Table 2. The Mws of all cellulose oxalates were approximately 40 kDa, which is only one tenth that of the original material. During the esterification, the molten oxalic acid dihydrate presumably acts as an extremely concentrated acid solution that catalyses the hydrolysis of cellulose. Nevertheless, due to the limited migration of the acid towards the ordered and crystalline regions, the hydrolysis should mostly occur in the less-ordered regions that are located at the surfaces of and within the fibrils or fibril aggregates. Moreover, it is interesting to see that the Mw increased slightly when the reaction time was prolonged from 15 min to 30 min and afterwards stabilised. As discussed above, the crosslinking of a few of the cellulose chains might be the reason for the increase of the Mw. The polydispersity index (PDI), except for COX60, gradually decreased from 11.1 to 3.1, suggesting the formation of a more homogenous material over longer reaction time.
| Sample | M w (kDa) | PDI |
|---|---|---|
| SDP | 392 | 11.1 |
| COX15 | 38 | 3.7 |
| COX30 | 44 | 3.5 |
| COX60 | 44 | 4.4 |
| COX120 | 41 | 3.1 |
The significantly decreased Mw as well as the considerable amount of free carboxyl groups suggests the possibility of disintegrating the cellulose powders in aqueous suspensions to prepare cellulose nanomaterials. Attempts were made to fibrillate COX30 and COX60 with the highest FCCs through sonication, and the supernatants were collected after centrifugation. By dynamic light scattering (DLS), the z-average of the nanoparticles in the suspension was 170 nm, which is in the length range of cellulose nanocrystals prepared from wood pulps.3
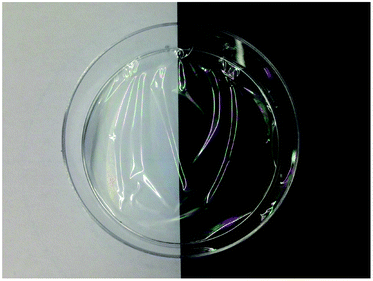
Transmission electron microscopy images shown in Fig. 1 confirmed the presence of nanostructured cellulose in the prepared suspension. The pH was adjusted to 3.5 to improve the dispersibility of the nanoparticles in dilute aqueous suspension.17 The shape of the nanocellulose was more similar to individual rods rather than fibrils, with an average length of 150–220 nm and an average width of 16–20 nm. When a concentrated suspension was observed between cross polarizers, birefringence patterns were present (Fig. 2), indicating an ordered arrangement of the cellulose nanocrystals.18 Moreover, it was possible to prepare transparent films through solvent casting of the suspension (Fig. 3). The films exhibited iridescence when tilted under light. The gravimetric yields for the conversion of cellulose oxalates into nanocrystals were 86.2% and 59.0%, as calculated from the dry weight of COX60 and COX30, respectively, while the overall gravimetric yields for the conversion of pulp/cellulose fibres into nanocrystals by using the current method were 80.6% (86.2% × 94%) and 58.3% (59.0% × 99%), as calculated from the dry weight of the softwood dissolving pulp.
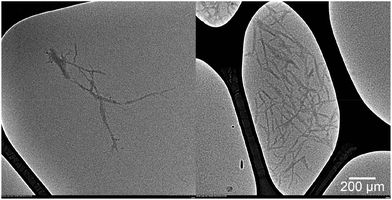 | ||
| Fig. 1 Transmission electron microscopy images of cellulose nanocrystals in dilute aqueous suspension. | ||
 | ||
| Fig. 2 A concentrated aqueous suspension (approximately 7 g l−1) of cellulose nanocrystals observed between crossed polarizers. | ||
The crystallinity indexes (CI) of the cast films from COX30 and COX60, as well as the reference dissolving pulp, were 68.2%, 74.5%, and 70.9% respectively. It is noteworthy that the CI of COX30-film was lower than that of the reference. Although the hydrolysis should have led to an increase of the CI as a result of the removal of less-ordered regions, the esterification of cellulose might make the cellulose chains locating at the surfaces of fibrils even less ordered, by introducing new functional groups that might interfere with the intermolecular interactions among the chains, which consequently results in a lower CI. The COX60-film has the highest CI among all the samples, which is presumably attributed to a more extensive removal of the less-ordered regions as a result of an extended hydrolysis. This is also supported by the lower gravimetric yield of COX60 than that of COX30, as shown in Table 1.
The described work demonstrates the possibility of preparing powder-shaped cellulose oxalates through a simple and solvent-free treatment of cellulose with molten oxalic acid dihydrate. A considerable amount of free carboxyl groups were introduced onto the surface of cellulose and the macromolecular structure of cellulose was broken down due to the acid-catalysed hydrolysis. By disintegrating the powder-shaped cellulose oxalates in aqueous suspension, carboxyl functionalised cellulose nanocrystals could be prepared. Compared to classic methods for the preparation of cellulose nanocrystals, the described method is simpler due to shortened procedures for purification, since no dialysis over several days is needed (see ESI†). On the other hand, the bulk reaction greatly improved the overall yield of the cellulose nanocrystals (81% versus 30–50%14) and reduced the extensive consumption of water that is often required in the classic method. Moreover, the described method also has a high potential of recycling both the unreacted oxalic acid dihydrate and the solvent used for washing the cellulose oxalates. All of these factors could contribute to reduce both the cost of production of cellulose nanocrystals and the negative environmental impact, and thereby may promote the market adaption of this renewable nanomaterial. It is noteworthy that, for analytical purposes, the prepared cellulose oxalates in this study were thoroughly washed by THF, which should be replaced by safer and more environmentally friendly solvents such as water and alcohols in the future.
Conflicts of interest
There are no conflicts to declare.References
- Y. Habibi, H. Chanzy and M. R. Vignon, Cellulose, 2006, 13, 679–687 CrossRef CAS.
- X. M. Dong, J. F. Revol and D. G. Gray, Cellulose, 1998, 5, 19–32 CrossRef CAS.
- S. Beck-Candanedo, M. Roman and D. G. Gray, Biomacromolecules, 2005, 6, 1048–1054 CrossRef CAS PubMed.
- B. G. Rånby, Discuss. Faraday Soc., 1951, 11, 158–164 RSC.
- A. F. Turbak, F. W. Snyder and K. R. Sandberg, Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential, United States, 1983 Search PubMed.
- M. Henriksson, G. Henriksson, L. A. Berglund and T. Lindstrom, Eur. Polym. J., 2007, 43, 3434–3441 CrossRef CAS.
- L. Wågberg, G. Decher, M. Norgren, T. Lindström, M. Ankerfors and K. Axnäs, Langmuir, 2008, 24, 784–795 CrossRef PubMed.
- T. Saito, S. Kimura, Y. Nishiyama and A. Isogai, Biomacromolecules, 2007, 8, 2485–2491 CrossRef CAS PubMed.
- S. Fujisawa, Y. Okita, H. Fukuzumi, T. Saito and A. Isogai, Carbohydr. Polym., 2011, 84, 579–583 CrossRef CAS.
- A. Isogai, T. Saito and H. Fukuzumi, Nanoscale, 2011, 3, 71–85 RSC.
- E. Abraham, B. Deepa, L. A. Pothan, M. Jacob, S. Thomas, U. Cvelbar and R. Anandjiwala, Carbohydr. Polym., 2011, 86, 1468–1475 CrossRef CAS.
- J. A. Sirvio, M. Visanko and H. Liimatainen, Biomacromolecules, 2016, 17, 3025–3032 CrossRef CAS PubMed.
- S. Spinella, A. Maiorana, Q. Qian, N. J. Dawson, V. Hepworth, S. A. McCallum, M. Ganesh, K. D. Singer and R. A. Gross, ACS Sustainable Chem. Eng., 2016, 4, 1538–1550 CrossRef CAS.
- L. Chen, J. Y. Zhu, C. Baez, P. Kitin and T. Elder, Green Chem., 2016, 18, 3835–3843 RSC.
- P. T. Larsson, E.-L. Hult, K. Wickholm, E. Pettersson and T. Iversen, Solid State Nucl. Magn. Reson., 1999, 15, 31–40 CrossRef CAS PubMed.
- Y. Pu, C. Ziemer and A. J. Ragauskas, Carbohydr. Res., 2006, 341, 591–597 CrossRef CAS PubMed.
- M. Kaushik, W. C. Chen, T. G. M. van de Ven and A. Moores, Nord. Pulp Pap. Res. J., 2014, 29, 77–84 CAS.
- X. M. Dong, T. Kimura, J. F. Revol and D. G. Gray, Langmuir, 1996, 12, 2076–2082 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, characterization and additional figures. See DOI: 10.1039/c7gc02489d |
| This journal is © The Royal Society of Chemistry 2017 |