Efficient eco-clean upgrading of isolated cellulose fibers by polyoxometalate (POM) catalyzed ozonation boosted by enzymes
Abstract
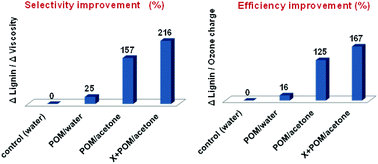
An extremely selective and effective delignification (bleaching) approach for upgrading of isolated cellulose fibers by ozone in the presence of mixed-addenda α-Keggin-type polyoxometalates (POM), such as heteropolyanions of series [PMo(12−n)VnO40](3+n)−, in aqueous solvent solutions has been developed, which is substantially superior to conventional bleaching techniques. Additional lignin removal (by 38.3%), improvement in brightness (by 16.5%), and the simultaneous increase in intrinsic viscosity (by 5.4%) observed after POM/ozonation of commercial eucalypt kraft pulp led to significant improvement in process selectivity and efficiency (by 157% and 125%, respectively) compared to common ozonation in water (with no catalyst and solvent). The intensification of POM catalysis by enzymatic pre-treatment of kraft pulp with highly specific xylanase preparations allowed further improving the selectivity and efficiency of POM-ozonation by ca. 60% and 40%, respectively. The integration of the POM/O3 stage into short totally chlorine-free bio-bleaching sequences, with hydrogen peroxide or/and alkali as the only additional reagents, made it possible to achieve the target properties of high grade cellulose fibers for commercial applications in a sustainable and ecologically friendly way.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...