Controlling silicone-saccharide interfaces: greening silicones†
Abstract
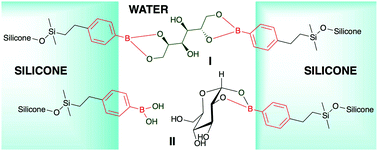
Silicone elastomers, which are normally crosslinked using metal catalysts, are traditionally reinforced with mineral fillers. We report that renewable saccharides can instead be used to both crosslink and reinforce silicones. The grafting of boronic acids to silicone polymers gives materials that, when added to aqueous solutions of mono- or polysaccharides, without catalysts, generated elastomers via the boronic acid interaction with saccharides. The efficiency of crosslinking, as shown by Young's moduli, depended strongly on the specific saccharide and the density of boronic acid groups on the silicone. Simple silicones normally phase separate in water saccharide mixtures. However, pretreatment of silicone boronates with the saccharide phytoglycogen, followed by exposure to water, led to stable aqueous phytoglycogen/silicone dispersions (pastes). The different outcomes arising from the order of addition are attributed to better dispersion of the silicone and saccharide in the latter case. Rheological studies of the pastes showed that, unlike the elastomers, viscosities depended more on the fraction of silicone in saccharide; number of boronic acid contact points between the silicone and saccharide was only a minor contributor. The equilibrium concentration of sugar/boronate contacts, which stabilize the water/oil interfaces, remains high even at high concentrations of water and even when the specific binding constant for an individual saccharide is low.
- This article is part of the themed collections: International Symposium on Green Chemistry 2017 and 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...