Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective reductive amination of aldehydes from nitro compounds catalyzed by molybdenum sulfide clusters†
E.
Pedrajas
 a,
I.
Sorribes‡
b,
K.
Junge
b,
M.
Beller
a,
I.
Sorribes‡
b,
K.
Junge
b,
M.
Beller
 *b and
R.
Llusar
*b and
R.
Llusar
 *a
*a
aDepartament de Química Física i Analítica, Universitat Jaume I, Av. Sos Baynat s/n, 12071 Castelló, Spain. E-mail: rosa.llusar@uji.es
bLeibniz-Institute für Katalyse e. V. an der Universität Rostock, Albert Einstein Str. 29a, 18059 Rostock, Germany
First published on 25th July 2017
Abstract
Secondary amines are selectively obtained from low value starting materials using hydrogen and a non-noble metal-based catalyst. The reductive amination of aldehydes from nitroarenes or nitroalkanes is efficiently catalyzed by a well-defined diamino molybdenum sulfide cluster in a one-pot homogeneous reaction. The integrity of the molecular cluster catalyst is preserved along the process.
Introduction
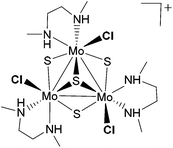
Amines are the most important building blocks employed in modern medicinal chemistry.1 Secondary amines are regularly prepared by the alkylation or reductive amination of the parent amines through transition metal, typically precious metals, catalyzed reactions.2,3 In the past few years, the catalytic alkylation of amines with alcohols under hydrogen-borrowing conditions4 as well as the catalytic reductive amination of carbonyl compounds using hydrogen as a reductant have been proposed as environmentally friendly procedures to produce N-substituted amines.5An actual challenge in catalysis research is to take low value starting materials and convert them into high value products using non-toxic earth abundant materials through an atom efficient procedure. Nitro compounds are an economic feedstock to provide primary amines and their use in reductive amination processes is highly attractive since prior isolation of the amine is not required. Our groups have recently shown that well-defined Mo3S4 cuboidal clusters are efficient catalysts for the chemoselective reduction of nitroarenes to anilines using different reducing agents.6–8 Interestingly, the diamino cluster of the formula [Mo3S4Cl3(dmen)3]+ (dmen = N,N′-dimethylethylenediamine), as depicted in Fig. 1, performs this transformation under mild conditions and using molecular hydrogen, the most “green” and least expensive reducing agent.6 On this basis, we became interested in its use as a catalyst for the related reductive amination of aldehydes with nitroarenes and nitroalkanes to generate secondary amines in a straightforward manner.
Domino catalytic transformations starting from nitroarenes and carbonyl compounds directed towards the preparation of N-alkylated aryl amines are not so common and they are usually done in the heterogeneous phase using palladium, platinum, rhodium or gold nanocatalysts.9–16 The N-alkylation of nitroarenes with aldehydes catalyzed by palladium operates under ambient hydrogen pressure at room temperature when alcohols are used as solvents. However, the formation of by-products such as benzylalcohol and toluene derivatives limits the selectivity of the process. Several cost-effective carbon-supported catalysts based on nanostructured Fe2O3 and Co-Co3O4 partially encapsulated by nitrogen-enriched graphene layers (Fe2O3/NGr@C and Co-Co3O4/NGr@C, respectively) have also been recently developed by some of us for the tandem reductive amination between nitro compounds and carbonyl compounds using hydrogen as a reductant.17–19 In general, these heterogeneous processes employing non-noble metals require more demanding conditions (110–170 °C and 50–70 bar H2) and contrary to the general assumption that anhydrous conditions favor reductive aminations, water has a positive influence on the reaction. Lanthanide metal organic frameworks have also proved to be active catalysts for the tandem reductive amination of nitrobenzene with heptanal to produce N-heptylaniline in moderate yields.20
Although heterogeneous catalysts are usually preferred for most industrial applications, molecularly defined complexes are still an attractive approach in catalysis due to their higher selectivity and easy modification. To our knowledge, the only examples of homogeneous catalysts capable of affording secondary amines from nitroarenes and aldehydes in the presence of hydrogen in a tandem fashion have been reported by Corma's group. The complex IrLCl (L = (E)-2-(tert-butyliminomethyl)-6-diisopropylaminomethyl-pyridine) is able to produce N-benzylaniline in 66% yield after the full conversion of nitrobenzene under 6 bar of H2 pressure and at 100 °C, while a trialkoxysilyl derivative of the pincer-type complex [RuHClCO((NHC)NN)] ((NHC)NN = (S)-1-((6-((3-aryl-2,3-dihydro-1H-imidazol-1-yl)methyl)pyridin-2-yl)methyl)) affords 86% conversion and 25% yield under similar conditions (80 °C and 5 bar H2).21,22 Remarkably, the immobilization of these molecular complexes in MOF architectures or mesoporous silica combines the catalytic power of the iridium or ruthenium complex with that of the support enhancing both conversion and selectivity.
Homogeneous catalysis by well-defined metal cluster complexes offers the possibility of performing transformations similar to those observed in the heterogeneous phase, which are known to be difficult to trace and control. Herein, we report the first homogeneous catalyst based on non-noble metals for the clean and atom efficient N-alkylation of amines starting from nitro compounds and aldehydes using molecular hydrogen as a benign reducing agent. The catalyst, a diamino cuboidal molybdenum sulfide cluster, is highly selective and preserves its integrity during the process.
Results and discussion
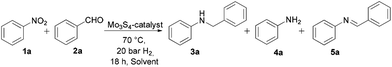
An initial evaluation of the [Mo3S4Cl3(dmen)3]+ catalyst performance on the model reaction of nitrobenzene (1a) with benzaldehyde (2a) was realized using different solvents motivated by the already established solvent influence on reactant conversion and product selectivity.17,19 For this study, we have tentatively chosen the optimum reaction conditions used for the hydrogenation of nitroarenes to anilines applying this molybdenum-based complex (18 h at 70 °C under 20 bar H2 and 5 mol% of the catalyst) and 1.2 equivalents of 2a have been added to the reaction mixture.6 While methanol appears as an optimum solvent for the hydrogenation of nitroarenes, the best yield towards the formation of the N-benzylaniline (3a) has been obtained in THF (Table 1, entries 1–3). When molecular sieves are added to remove water from the reaction mixture and tentatively shift the equilibrium towards the in situ generated imine, the conversion of 1a is halved and no secondary amine 3a is formed (Table 1, entry 4). Surprisingly, similar reactivity is observed in THF–H2O mixtures (Table 1, entries 5 and 6). Thus, traces of water, approximately between 150 and 1500 ppm, are required to achieve a quantitative formation of 3a, while an excess of water has a detrimental effect on the reaction outcome (Table 1, entry 4). These results suggest a direct implication of the water molecules in the reaction mechanism. The beneficial effect of a controlled amount of water in the reductive N-alkylation of amines is not unprecedented.23 In contrast, larger amounts of water, up to 50%, are tolerated in the tandem reactions of nitro compounds to secondary amines catalyzed by Co-Co3O4/NGr@C and Fe2O3/NGr@C catalysts in THF.17–19 In this latter case, water addition is believed to promote the hydrophobic association of aldehydes and amines as well as suppress catalyst poisoning.24| Entrya | Solvent | Conv.b [%] | Yieldb [%] | ||
|---|---|---|---|---|---|
| 3a | 4a | 5a | |||
| a Reaction conditions: 1a (0.1 mmol), 2a (0.12 mmol), H2 (20 bar), catalyst (5 mol%), solvent (2 mL), 18 h, 70 °C. b Determined by GC analysis using hexadecane as an internal standard. | |||||
| 1 | 1,4-Dioxane | 31 | 0 | 7 | 22 |
| 2 | CH3OH | 99 | 74 | 23 | 0 |
| 3 | THF (150–1500 ppm of H2O) | >99 | 99 | 0 | 0 |
| 4 | THF (molecular sieves) | 50 | 0 | 18 | 30 |
| 5 | THF–H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
47 | 0 | 36 | 11 |
| 6 | THF–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
14 | 0 | 11 | 2 |
Next, the optimization of the temperature, hydrogen pressure and catalyst loading were addressed (Tables SI1–SI3†). A full conversion of 1a with a quantitative yield of 3a (>99%) occurs at 70 °C, 20 bar of hydrogen pressure and 5 mol% of catalyst loading within 18 hours. In contrast, a partial conversion of 1a (48%) with only a scarce formation of 4a (7% yield) and 5a (38% yield) is achieved after 4 h. Then, longer reaction times are required for the reduction of the imine intermediate, which is in good agreement with the general tendency that imine hydrogenation is regarded as the rate-determining step in the reductive amination sequence. It should be noted that when aldehydes were replaced by ketones, the reaction stopped at the aniline formation stage and no traces of the iminium ion or the secondary amine could be detected.
Our recent studies on the catalytic reduction of nitroarenes to aniline derivatives mediated by Mo3S4-based clusters decorated with diphosphino, diamino or diimino ligands show no fragmentation of the cluster core during the first stage of this tandem process.6–8 Here, a color change, from green to brown, occurs during the catalytic process due to the basicity increase caused by the amine generation. However, analysis of the reaction mixture by electrospray ionization mass spectrometry (ESI-MS) shows a single peak at m/z = 786.9, associated with the pseudomolecular [Mo3S4Cl3(dmen)3]+ ion on the basis of the m/z value and its characteristic isotopic pattern (Fig. SI1†). Additionally, cluster integrity was further confirmed by 1H NMR spectroscopy (Fig. SI2†). Therefore, the integrity of the cluster core is also preserved during the reductive amination stage. In addition, catalyst recycling in the model reaction after recovering the catalyst by evaporating, washing and drying the reaction mixture shows full conversion and an excellent yield of 3a (90%) in the second run (Fig. SI3†). In the third run, the conversion decreased to 67% and only traces of the secondary amine 3a were formed together with N-benzylideneaniline (5a) as a major product (46%) and aniline (4a) in 16% yield.
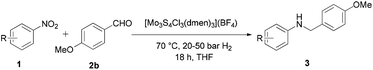
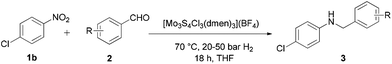
To prove the general applicability of the Mo3S4-based catalytic system, the reactions between various functionalized nitro compounds and aldehydes were investigated. More specifically, different nitro compounds were reacted with p-anisaldehyde (2b) (Table 2), and as shown in Table 3, the reductive amination of diverse structure aldehydes was carried out with p-chloronitrobenzene (1b). To our delight, the corresponding secondary amines were afforded in good to excellent yields. As a general trend, when both the aldehydes and nitroarenes are functionalized with electron-donating groups, such as alkyl, alkoxy or thiomethyl groups, the one-pot reductive amination reaction proceeds smoothly towards the formation of the secondary amines. However, the presence of electron-withdrawing groups on the aromatic ring had a high impact on the reductive amination activity since an increased hydrogen pressure (up to 50 bar H2) is required to avoid the accumulation of the imine intermediate.
| Entrya | Substrate | P (bar) | Conv.b [%] | Yield amine 3b [%] |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.25 mmol), 2b (0.3 mmol), catalyst (6 mol% related to nitroarene), solvent (2 mL). b Determined by GC analysis using hexadecane as an internal standard; yields of the isolated products are given in parentheses. c Traces of aniline as the by-product. d Traces of imine as the by-product. e 24 h. f 100 °C of temperature. g 150 °C of temperature; yield based on 1H NMR using 2,4,6-trimethylphenol as an internal standard. | ||||
| 1 | R = H | 20 | >99 | (98) |
| 2c | R = 3-Me | 20 | >99 | 91 |
| 3d | R = 3-CF3 | 50 | >99 | (97) |
| 4 | R = 4-F | 20 | >99 | >99 |
| 5d | R = 4-Cl | 20 | >99 | (91) |
| 6 | R = 4-I | 20 | >99 | (90) |
| 7d,e | R = 4-CN | 50 | >99 | (82) |
| 8d,e | R = 4-COOMe | 50 | >99 | (85) |
| 9d,f |
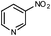
|
50 | >99 | (81) |
| 10c,d,g |

|
50 | >99 | 61 |
| Entrya | Substrate | P (bar) | Conv.b [%] | Yield amine 3b [%] |
|---|---|---|---|---|
| a Reaction conditions: 1b (0.25 mmol), 2 (0.3 mmol), catalyst (6 mol% related to nitroarene), solvent (2 mL). b Determined by GC analysis using hexadecane as an internal standard; yields of the isolated products are given in parentheses. c 24 h. d Traces of imine as the by-product. | ||||
| 1 | R = H | 40 | >99 | (95) |
| 2c | R = 4-iPr | 20 | >99 | (90) |
| 3 | R = 3-OMe; 4-OEt | 20 | >99 | (97) |
| 4 | R = 4-SMe | 20 | >99 | (95) |
| 5 | R = 4-F | 20 | >99 | >99 |
| 6c,d | R = 2-F | 50 | >99 | (68) |
| 7c,d | R = 4-Br | 50 | >99 | (77) |
| 8d | R = 3-CHCH2 | 40 | >99 | (85) |
| 9c,d |
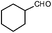
|
50 | >99 | 88 (79) |
Gratifyingly, both halogen-substituted nitroarenes and aldehydes react to give the corresponding halogenated secondary amines in moderate to excellent yields without any dehalogenation processes. Notably, the reductive amination of p-fluorobenzaldehyde with 1b affords a quantitative yield of the expected alkylated amine (Table 3, entry 5). However, the reaction with the ortho-isomer requires an increased hydrogen pressure (50 bar H2) to obtain a moderate yield of the corresponding secondary amine (Table 3, entry 6). Hence, it seems that reactivity could be affected by both electronic and steric effects.
From a synthetic point of view, substrates bearing reducible functionalities are highly attractive. In this respect, we tested some substrates functionalized with nitriles, esters or olefines. To our delight, these moieties were well tolerated, thus furnishing the expected amines in 82–85% isolated yields (Table 2, entries 7 and 8; Table 3, entry 8). In addition, a nitro-substituted heteroarene was tested and the corresponding N-heterocyclic amine was isolated in 81% yield (Table 2, entry 9).
Nowadays, the reductive amination of aldehydes with aliphatic nitro compounds remains an important challenge. Interestingly, in the presence of our Mo3S4-based catalyst the reaction between 2b and 1-nitrohexane leads to the formation of the secondary amine in 61% yield (Table 2, entry 10). Moreover, our catalytic system also works with cyclic aliphatic aldehydes. The reductive amination of cyclohexanecarboxaldehyde with 1b affords a good yield (88%) of the desired amine (Table 3, entry 9).
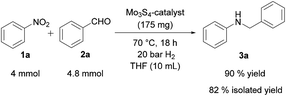
Finally, the preparative value of this protocol was further demonstrated by upscaling the model reaction of nitrobenzene (1a) with benzaldehyde (2a) by a factor of 40 and using a considerably higher concentration of reactants. As shown in Scheme 1, N-benzylaniline (3a) was obtained in 82% yield after purification by column chromatography.
Conclusions
In conclusion, we have developed an atom-efficient catalytic protocol for the synthesis of secondary amines through a one-pot reductive amination reaction from easily available nitroarenes and aldehydes using hydrogen as a reducing agent. The use of a well-defined diamino molecular Mo3S4 cluster as a catalyst allows for the successful reductive amination of aldehydes with the in situ generated primary amines selectively affording a variety of secondary amines in good to excellent yields. Remarkably, this procedure applies to aromatic as well as to aliphatic nitro compounds or aldehydes. Spectrometric and spectroscopic techniques show no changes in cluster composition or evidence of cluster fragmentation during the catalytic reaction. Mechanistic investigations of the catalytic process which combine theory with experiment are in progress.Experimental section
Synthesis of the catalyst
The catalyst [Mo3S4Cl3(dmen)3](BF4) was prepared starting from the [Mo3S4(tu)8(H2O)]Cl4·4H2O thiourea precursor according to the literature.6General procedure for the one-pot reductive amination
An 8 mL glass vial containing a stirring bar was sequentially charged with the molybdenum catalyst (4.4 mg, 0.005 mmol of [Mo3S4Cl3(dmen)3](BF4)), nitrobenzene (10 μL, 0.097 mmol), benzaldehyde (12 μL, 0.12 mmol), n-hexadecane (15 μL; added as an internal standard) and 2 mL of THF. Afterwards, the reaction vial was capped with a septum equipped with a needle and set in the alloy plate, which was then placed into a 300 mL autoclave. Once sealed, the autoclave was purged three times with 30 bar of hydrogen, then pressurized to 20 bar and placed into an aluminum block, which was preheated at 70 °C. After 18 h, the autoclave was cooled to room temperature and the hydrogen was released. Ethyl acetate (2 mL) was then added, and a sample was taken to be analyzed using GC. To determine the isolated yields of the anilines, the general procedure was scaled up by the factor of 2.5, and no internal standard was added. After completion of the reaction, the mixture was purified by silica column chromatography (n-heptane/ethyl acetate mixtures) to give the corresponding anilines. In the case of the nitroalkane, 2,4,6-trimethylphenol was added as an internal standard and the yield was calculated based on 1H NMR data.Acknowledgements
The financial support of the Spanish Ministerio de Economía y Competitividad (Grant CTQ2015-65207-P) and Generalitat Valenciana (PrometeoII/2014/022) is gratefully acknowledged. The authors also thank the Serveis Centrals d'Instrumentació Cientifica (SCIC) of the Universitat Jaume I for providing them with mass spectrometry and NMR infrastructure. E. Pedrajas thanks the University Jaume I for a predoctoral fellowship.References
- S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS PubMed.
- G. Guillena, D. J. Ramón and M. Yus, Chem. Rev., 2010, 110, 1611–1641 CrossRef CAS PubMed.
- E. W. Baxter and A. B. Reitz, in Organic Reactions, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2002, pp. 1–714 Search PubMed.
- R. Kawahara, K. Fujita and R. Yamaguchi, Adv. Synth. Catal., 2011, 353, 1161–1168 CrossRef CAS.
- S. Fleischer, S. Zhou, K. Junge and M. Beller, Chem. – Asian J., 2011, 6, 2240–2245 CrossRef CAS PubMed.
- E. Pedrajas, I. Sorribes, A. L. Gushchin, Y. A. Laricheva, K. Junge, M. Beller and R. Llusar, ChemCatChem, 2017, 9, 1128–1134 CrossRef CAS.
- E. Pedrajas, I. Sorribes, K. Junge, M. Beller and R. Llusar, ChemCatChem, 2015, 7, 2675–2681 CrossRef CAS.
- I. Sorribes, G. Wienhöfer, C. Vicent, K. Junge, R. Llusar and M. Beller, Angew. Chem., Int. Ed., 2012, 51, 7794–7798 CrossRef CAS PubMed.
- B. Sreedhar, P. S. Reddy and D. K. Devi, J. Org. Chem., 2009, 74, 8806–8809 CrossRef CAS PubMed.
- L. Li, Z. Niu, S. Cai, Y. Zhi, H. Li, H. Rong, L. Liu, L. Liu, W. He and Y. Li, Chem. Commun., 2013, 49, 6843 RSC.
- S. Wei, Z. Dong, Z. Ma, J. Sun and J. Ma, Catal. Commun., 2013, 30, 40–44 CrossRef CAS.
- B. Sreedhar and V. S. Rawat, Synth. Commun., 2012, 42, 2490–2502 CrossRef CAS.
- L. Hu, X. Cao, D. Ge, H. Hong, Z. Guo, L. Chen, X. Sun, J. Tang, J. Zheng, J. Lu and H. Gu, Chem. – Eur. J., 2011, 17, 14283–14287 CrossRef CAS PubMed.
- Y. Yamane, X. Liu, A. Hamasaki, T. Ishida, M. Haruta, T. Yokoyama and M. Tokunaga, Org. Lett., 2009, 11, 5162–5165 CrossRef CAS PubMed.
- A. L. Nuzhdin, E. A. Artiukha, G. A. Bukhtiyarova, S. Y. Zaytsev, P. E. Plyusnin, Y. V. Shubin and V. I. Bukhtiyarov, RSC Adv., 2016, 6, 88366–88372 RSC.
- L. Huang, Z. Wang, L. Geng, R. Chen, W. Xing, Y. Wang and J. Huang, RSC Adv., 2015, 5, 56936–56941 RSC.
- T. Stemmler, F. A. Westerhaus, A.-E. Surkus, M.-M. Pohl, K. Junge and M. Beller, Green Chem., 2014, 16, 4535–4540 RSC.
- R. V. Jagadeesh, T. Stemmler, A.-E. Surkus, H. Junge, K. Junge and M. Beller, Nat. Protoc., 2015, 10, 548–557 CrossRef CAS PubMed.
- T. Stemmler, A.-E. Surkus, M.-M. Pohl, K. Junge and M. Beller, ChemSusChem, 2014, 7, 3012–3016 CrossRef CAS PubMed.
- R. F. D'Vries, M. Iglesias, N. Snejko, S. Alvarez-Garcia, E. Gutiérrez-Puebla and M. A. Monge, J. Mater. Chem., 2012, 22, 1191–1198 RSC.
- M. Pintado-Sierra, A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sánchez, J. Catal., 2013, 299, 137–145 CrossRef CAS.
- C. del Pozo, A. Corma, M. Iglesias and F. Sánchez, J. Catal., 2012, 291, 110–116 CrossRef CAS.
- J. R. Cabrero-Antonino, R. Adam, K. Junge and M. Beller, Catal. Sci. Technol., 2016, 6, 7956–7966 CAS.
- S. Sato, T. Sakamoto, E. Miyazawa and Y. Kikugawa, Tetrahedron, 2004, 60, 7899–7906 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: General information, advanced results and analytical data. See DOI: 10.1039/c7gc01603d |
| ‡ Present address: Instituto de Tecnología Química, Universitat Politècnica de València-Consejo Superior de Investigaciones Científicas, Av. De los Naranjos s/n, 46022 Valencia, Spain. |
| This journal is © The Royal Society of Chemistry 2017 |