An in situ formed Ca2+–crown ether complex and its use in CO2-fixation reactions with terminal and internal epoxides†
Abstract
Herein we report an efficient catalytic system based on readily available calcium iodide and 18-crown-6 ether for the atom economical addition of CO2 to epoxides. 1H NMR experiments revealed the selective in situ formation of a crown ether complex. This catalyst allows the conversion of various terminal epoxides under 1 atm CO2 pressure even at room temperature. Remarkably, a broad range of internal epoxides with various substitution patterns and substituents were smoothly converted which confirms the high efficiency and capability of the protocol. Notably, most of the internal carbonates were synthesized in high yields and diastereoselectivities of up to ≥99%. Furthermore, this system operates under solvent-free conditions without any co-catalysts e.g. onium salts.
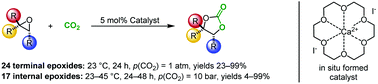
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...