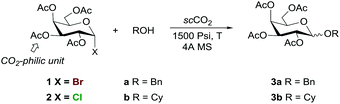Open Access Article
Open Access ArticleMetal-free and VOC-free O-glycosylation in supercritical CO2†
Adrià
Cardona
 ,
Omar
Boutureira
,
Omar
Boutureira
 ,
Sergio
Castillón
,
Sergio
Castillón
 ,
Yolanda
Díaz
,
Yolanda
Díaz
 * and
M. Isabel
Matheu
* and
M. Isabel
Matheu
 *
*
Departament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/ Marcel.lí Domingo 1, 43007, Tarragona, Spain. E-mail: maribel.matheu@urv.cat; yolanda.diaz@urv.cat
First published on 3rd May 2017
Abstract
Supercritical carbon dioxide (scCO2) is a suitable medium to perform transition metal-free glycosylation reactions in the absence of volatile organic solvents (VOCs) using glycosyl halides as glycosyl donors. The methodology described here can be applied for obtaining O-glycosides in a totally green reaction, as well as orthoesters, depending on the reaction conditions. The process is much more sensitive to temperature changes than to pressure modification, with glycosyl chlorides requiring higher temperatures to be activated than glycosyl bromides. Pivaloyl groups act as good CO2-philic units and are shown to be the best choice to obtain good stereoselectivities. The relevance of the fluid nature and supercritical conditions was also evidenced.
Introduction
Glycoconjugates have been of special interest in recent years because of the vital role that carbohydrates play in biological recognition processes, such as host–pathogen interaction, cell adhesion, and development, among others.1,2 The key step in the synthesis of glycoconjugates is the glycosylation reaction, which links a carbohydrate or oligosaccharide with a lipid or a protein.3Since the establishment of its foundation by Michael4 and Fischer,5 a large number of glycosylation methods have been described.6 Nevertheless, the complexity and diversity of glycoconjugates (branched structures, different anomeric configurations, several possible regioisomers, and a variety of monosaccharides) in relation to the relatively simple sequences of other biopolymers (proteins and nucleic acids) demand the use of efficient synthesis methods and limit automated methodologies.
All elements contributing to the glycosylation reaction affect the incidence and selectivity of the process: glycosyl acceptor and donor, protecting and leaving groups, promoter, solvent, temperature, etc. However, the conjunction leaving group/activator-promoter is a key issue for the success of the reaction and hence, there have been continuous efforts to develop novel leaving groups and new promoter/catalyst pairs in order to enhance the glycosylation efficiency. In most of the cases, strong Brønsted or Lewis acids, alkylating agents, metallic salts or transition metal complexes are required. This fact implies the elimination of such promoters at the end of the reaction (Scheme 1).
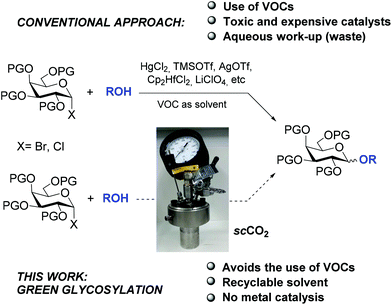 | ||
| Scheme 1 Conventional approach for the glycosylation from glycosyl halides and our proposal using scCO2. | ||
In spite of the increasing interest in green chemical processes, few efforts have been made for obtaining glycoconjugates through a green glycosylation reaction.7 In contrast, the main promoters for this reaction are toxic, expensive and often light- and moisture-sensitive,8 especially when glycosyl halides are used as donors. Another important aspect in the research towards more sustainable processes in chemical synthesis is the replacement of volatile organic compounds (VOCs) as solvents.9 However, in the glycosylation reaction, solvents play a critical role in terms of stabilizing the corresponding intermediates, and in the α/β selectivity of the product. Thus, the choice of solvent is a strategic parameter in glycosylation reactions and, in this regard, “green” solvents have been scarcely used, an organic solvent being usually the first and only choice.
In this respect, supercritical carbon dioxide (scCO2) has emerged as an attractive non-toxic, low cost, abundant and easy to recycle green solvent.10 Furthermore, scCO2 is easily accessed (Tc = 31.1 °C, Pc = 72.9 atm, 1071,33 Psi)11 and can be removed after the reaction by simple depressurization. For these reasons, the interest in using this solvent has increased exponentially in recent years.12
scCO2 was considered as a nonpolar solvent mainly because of its low dielectric constant and zero molecular dipole moment. However, it has been claimed that its charge separation and significant bond dipoles producing a quadrupole allow it to act as a weak Lewis acid or Lewis base.10,13 This microscopic view explains the significant site-specific solute–solvent interactions that it can establish, underscoring the polar nature of this solvent. Indeed, scCO2 can solubilize nonpolar and polar compounds. On the other hand, many biologically interesting products, such as carbohydrates, are highly polar and tend to exhibit low solubility in scCO2. However, acetylation of hydroxy groups has been shown to be an efficient strategy to increase their solubility in scCO2.14 This fact points out the possible use of scCO2 as a promising solvent in glycosylation reactions. In this regard, Hinou and Nishimura described an efficient sulphated-zirconia-promoted glycosylation starting from acetylated sugars in scCO2.15
Furthermore, it has been recently described that scCO2 promotes the heterolysis of carbon–halogen bonds in aromatic systems with good results in Friedel–Crafts type reactions,16 which was justified by the cluster effect of scCO2 in polar solute molecules.13,17,18 Taking into account the fact that the heterolysis of an activated glycosidic bond is the initial step for the glycosylation reaction and considering that, similar to Friedel–Crafts reactions, a cation is the most common intermediate in this reaction, we hypothesized that the glycosylation reaction starting from glycosyl halides could be performed in scCO2 and, more interestingly, in the absence of a promoter, additionally avoiding the use of VOCs (Scheme 1).
Results and discussion
Due to the reported solubility of polyacetylated systems in scCO2, and the fact that D-galactose is a common carbohydrate found in glycoconjugates, tetra-O-acetyl-α-D-galactopyranosyl halides were chosen as glycosyl donors for this study. The preliminary experiments using benzyl alcohol with different galactosyl halides (Cl, Br, I) at 1500 Psi and temperatures ranging between 40 and 90 °C led to the corresponding O-glycosides with a varying degree of success, the corresponding chlorides and bromides being optimal in terms of balance between reactivity and practical handling. Penta-O-acetyl-β-D-galactopyranose was also explored as a glycosyl donor but all attempts of working at 1500 Psi led to the recovery of the unaltered starting material, despite using temperatures as high as 100 °C. Thus, the glycosylation conditions were optimised using tetra-O-acetyl-α-D-galactopyranosyl bromide 1 and tetra-O-acetyl-α-D-galactopyranosyl chloride 2 as glycosyl donors as well as benzyl alcohol (a) and cyclohexanol (b) as acceptors. The results are summarised in Table 1.| Entrya | Glycosyl donor | Alcohol | T (°C) | ROH (equiv.) | t (h) | Conv.b (%) | Yieldc (%) |
|---|---|---|---|---|---|---|---|
a Galactosyl bromide 1 or 2 (0.365 mmol) and benzyl alcohol (a) or cyclohexanol (b) were allowed to react in the presence of 4 Å MS (ca. 100 mg) in a stainless steel reactor which was filled with CO2 until 1500 Psi, unless stated.
b Conversion was determined by 1H NMR spectroscopy.
c Isolated yields.
d The reaction was performed at P = 1200 Psi.
e No reaction.
f The reaction was performed at P = 3300 Psi.
g Complex mixture.
h α/β ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. 1.2.
|
|||||||
| 1d | 1 | a | 40 | 4 | 3 | n.r.e | — |
| 2 | 1 | a | 40 | 4 | 14 | n.r. | — |
| 3 | 1 | a | 60 | 4 | 14 | >98 | 63 |
| 4 | 1 | a | 60 | 1.4 | 3 | 77 | 40 |
| 5 | 1 | a | 60 | 1.4 | 5 | 89 | 49 |
| 6 | 1 | a | 60 | 1.4 | 14 | 95 | 58 |
| 7 | 2 | b | 60 | 4 | 14 | 12 | n.d. |
| 8f | 2 | b | 60 | 4 | 14 | 12 | n.d. |
| 9 | 2 | b | 60 | 4 | 110 | 30 | n.d. |
| 10 | 2 | b | 75 | 4 | 14 | 15 | n.d. |
| 11 | 2 | b | 80 | 4 | 14 | 20 | n.d. |
| 12 | 2 | b | 100 | 4 | 14 | >98g | n.d. |
| 13 | 2 | b | 90 | 4 | 14 | 73 | n.d. |
| 14 | 2 | b | 90 | 4 | 24 | >95h | 73 |
The glycosylation of benzyl alcohol (a) with tetra-O-acetyl-α-D-galactopyranosyl bromide 1 using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a molar ratio of 1
a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 showed that the reaction required minimum pressure and temperature values of 1500 Psi and 60 °C, respectively, to proceed (Table 1, entries 1, 2 vs. 3). Lowering the excess acceptor concentration to 1.4 equiv. resulted in almost the same conversion and glycoside yield (Table 1, entry 6), which could be especially important when high value acceptors are concerned. Shorter reaction times were detrimental for the yield and conversion (Table 1, entry 6 vs. entries 4 and 5). In some cases (results not shown), variable amounts of the lactol or the glycoside unprotected at position C-2 were obtained, which were reduced by adding 4 Å MS to the reaction mixture.
4 showed that the reaction required minimum pressure and temperature values of 1500 Psi and 60 °C, respectively, to proceed (Table 1, entries 1, 2 vs. 3). Lowering the excess acceptor concentration to 1.4 equiv. resulted in almost the same conversion and glycoside yield (Table 1, entry 6), which could be especially important when high value acceptors are concerned. Shorter reaction times were detrimental for the yield and conversion (Table 1, entry 6 vs. entries 4 and 5). In some cases (results not shown), variable amounts of the lactol or the glycoside unprotected at position C-2 were obtained, which were reduced by adding 4 Å MS to the reaction mixture.
At first sight, experiments from less reactive donor 2 were anticipated to be more challenging and therefore cyclohexanol, a more demanding acceptor, was used as a model for the reaction conditions optimization. Unlike benzyl alcohol, the use of 1.4 equiv. of cyclohexanol resulted in incomplete conversion (results not shown); hence, for the sake of exploratory purposes, reactions further explored with 2 used 4 equiv. of the acceptor. Under the optimized conditions set for galactosyl bromide 1 (Table 1, entry 3), the reaction of galactosyl chloride 2 led to a poor 12% conversion (Table 1, entry 7), which did not improve by increasing the pressure (Table 1, entry 8). As expected, the reaction from 2 proved slower than that from glycosyl bromide 1 and after 110 h only 30% conversion was achieved (Table 1, entry 9). As already observed with 1, the reaction is much more sensitive to temperature than to pressure modification.
Thus, galactosyl chloride 2 required higher temperatures to be activated in scCO2 than its bromide counterpart, the optimal conditions being 90 °C and 1500 Psi (Table 1, entry 14 vs. entries 10–13). These results open the way for exploring the possibility of performing orthogonal glycosylations in scCO2 in the future.
Encouraged by these results, the study was also extended to differently acylated galactosyl bromides 1, 4, and 5, chlorides 2 and 6 and glycosyl acceptors a–c (Scheme 2). Taking into account the fact that the progress of the reaction cannot be monitored when working with a pressure reactor and that we were in an exploratory stage, we decided to use 4 equiv. of the acceptor, essentially to guarantee full conversion. Tetraacetylated galactosyl bromide 1 afforded moderately good isolated yields of glycosides 3a–c (65–85%), with moderate α/β selectivities, which were similar in all acceptors (Scheme 2).
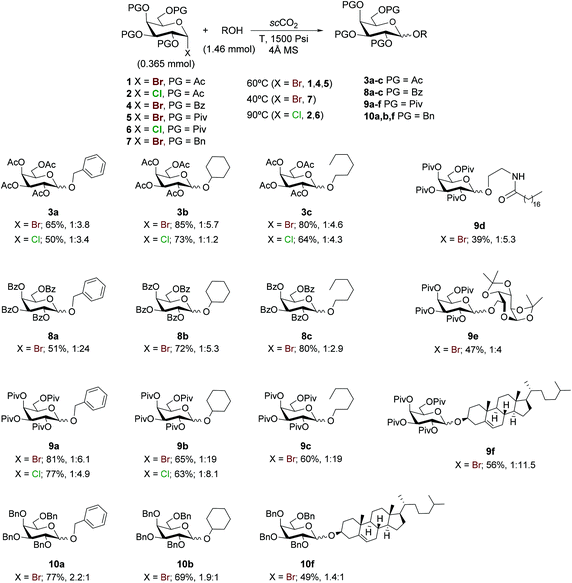 | ||
| Scheme 2 Scope of the glycosylation in scCO2. Conversions of the galactosyl donor are >98% except for 8a (88%). Isolated yield and α/β ratio values are shown. | ||
Reactions of benzoyl- and pivaloyl-protected galactosyl bromides 4 and 5 required longer reaction times. For practical reasons the reaction mixtures were allowed to stir for 24 h to guarantee full conversion. Nevertheless, control experiments with 5 and b showed that the reaction can proceed at shorter reaction times (12 h).
Higher temperatures (75–85 °C) led to lower yields and stereoselectivities due to the decomposition of the galactosyl donor (results not shown). The results from benzoylated galactosyl donor 4 (Scheme 2) to afford glycosides 8b and 8c were parallel to those obtained from 1, except for benzyl alcohol, which rendered 8a in a modest yield but excellent stereoselectivity (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 α/β ratio). The better stereoselectivities (from 1
24 α/β ratio). The better stereoselectivities (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.1 to 1
6.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 α/β ratio) were achieved by using the more hindered tetra-O-pivaloyl protected galactosyl bromide 5.
19 α/β ratio) were achieved by using the more hindered tetra-O-pivaloyl protected galactosyl bromide 5.
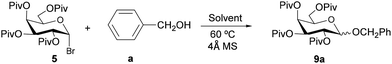
In order to explore the preparation of higher amounts of the material, a five-fold more concentrated reaction using 5 as the glycosyl donor with cyclohexanol (b) as the acceptor was performed. The reaction proceeded with full conversion leading to glycoside 9b in similar yields (62%) although with a slight decrease in stereoselectivity (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 α/β ratio) compared to the experiment performed at a lower concentration (Scheme 2). The stereoselectivity dependence on the concentration has already been observed and could be explained by the supramolecular aggregation in the reaction mixture leading to supramers.19
9 α/β ratio) compared to the experiment performed at a lower concentration (Scheme 2). The stereoselectivity dependence on the concentration has already been observed and could be explained by the supramolecular aggregation in the reaction mixture leading to supramers.19
Acetyl- and pivaloyl-protected galactosyl chlorides 2 and 6 were treated with benzyl alcohol, cyclohexanol and 1-hexanol, under the optimized glycosylation conditions for glycosyl chlorides to afford the corresponding glycosides 3a–c and 9a–b, respectively in moderate to good yields (50–77%). The stereoselectivities were comparable to those obtained from the galactosyl bromide 1, with the exception of cyclohexyl tetra-O-acetyl-D-galactopyranoside 3b, which led to almost null stereoselectivity. In the reactions performed with glycosyl chloride 2 the stereoselectivity decreases when the bulkiness or the basicity of the alcohol acceptor increases.
In light of the results obtained from the differently protected galactosyl donors 1, 2, 4, 5 and 6, the ester groups tested in this study appear to be good CO2-philic units.
A selection of interesting acceptors such as the lipidic amidoalcohol d, the protected carbohydrate e and cholesterol (f) were glycosylated with 5 to afford glycolipid 9d, disaccharide 9e, and chloresteryl galactoside 9f in yields ranging from 39% to 56% and α/β selectivities from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.5 (9f) to 1
11.5 (9f) to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (9e).
4 (9e).
In general, appreciable deviations of isolated yield values with respect to full conversions are observed. The decrease in yield is explained by the formation of the corresponding lactol as a secondary product. The most significant divergences were obtained when less reactive glycosyl acceptors were employed (reactions leading to 9d–f), where 1,3,4,6-tetra-O-pivaloyl-α-D-galactopyranose was also observed as a side product in the crude spectra.20
The reaction with the more activated ether-protected glycosyl donor 7 leading to compounds 10a, 10b, and 10f proceeded at a lower temperature (40 °C) and lower reaction time (3 h) except for 10f, which required 12 h to complete, as expected from the lower reactivity of cholesterol. It is noteworthy that good yields (ca. 70%) were obtained from these highly reactive galactosyl donors, except for 10f, which was obtained in a moderate 49% yield due to the competitive formation of the lactol as a secondary product. This result is in line with the lower yields typically reported for cholesterol compared with more simple acceptors. In these cases the α-anomer was the major product obtained, as expected.
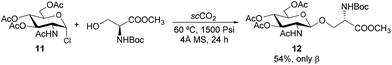
Our methodology also proved efficient for the challenging glycosylation of a protected serine derivative with commercially available 2-acetamidoglucosyl chloride 11, which afforded O-glycoside 12 in 54% yield as the sole anomer together with a glycal side-product. Milder conditions were required in this case due to the higher reactivity of glycosyl donor 11 (Scheme 3).
As shown, all experiments undertaken in this study (Scheme 2) led to the formation of variable amounts of α-glycoside as a minor product as well as the expected β-anomer, despite the fact that glycosyl donors with participating groups at position 2 were used. The α-anomer could directly arise from the trapping of the oxonium intermediate.21 Alternatively, its formation could also be explained by the trapping of the acetoxonium ion to give the β anomer followed by a HBr-promoted anomerization process.22
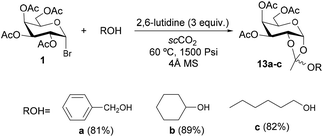
To address the origin of the α/β stereoselectivity, the reaction of donor 1 with acceptor a was performed in the presence of excess lutidine as an acid scavenger.23 Under these conditions, no glycoside was observed but instead, ortho-ester 13a was exclusively obtained in 81% yield. Similar results were obtained from acceptors b and c (Scheme 4). These experiments open the door to the use of this methodology for the synthesis of orthoesters, which are valuable starting materials in oligosaccharide synthesis.23,24
Still, some issues remained uncertain. Does the α/β mixture arise from direct trapping of the oxonium/acetoxonium intermediate or from subsequent HBr-promoted anomerization? Conducting experiments at different reaction times would indeed shed some light on this issue. A set of experiments with galactosyl donor 1 and cyclohexanol under the optimized conditions at 2 h, 5 h and 14 h led to conversion–(α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) values of 50%–(1
β) values of 50%–(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.7), 89%–(1
5.7), 89%–(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.1) and >98%–(1
6.1) and >98%–(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.3), respectively. Obtaining essentially the same α/β ratio regardless of the conversion would suggest that the α anomer results from direct trapping of the onium intermediate, and it is not generated in a subsequent anomerization.
4.3), respectively. Obtaining essentially the same α/β ratio regardless of the conversion would suggest that the α anomer results from direct trapping of the onium intermediate, and it is not generated in a subsequent anomerization.
Furthermore, when cyclohexyl tetra-O-acetyl-β-D-galactopyranoside (obtained by purification of the previous α/β 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.7 mixture, Scheme 2) was left to stir in scCO2 under the standard conditions (1500 Psi and 60 °C) for 14 h, intact β-galactoside was recovered after depressurization, ruling out an anomerization process promoted by the supercritical fluid.
5.7 mixture, Scheme 2) was left to stir in scCO2 under the standard conditions (1500 Psi and 60 °C) for 14 h, intact β-galactoside was recovered after depressurization, ruling out an anomerization process promoted by the supercritical fluid.
Moreover, the presence of molecular sieves has been described as a neutral acid scavenger in some reactions and, in particular, in the glycosylation reaction and, in this respect, could alter the stereoselectivity outcome of the reaction.25 In our experiment, the use of molecular sieves did not produce a change in the stereoselectivity of the glycosylation but, as mentioned earlier, proved beneficial in minimizing the formation of hydrolysis byproducts.
To rule out that the activation of galactosyl halides might be exclusively due to a temperature effect, a set of different assays were conducted from galactosyl bromide 5 without any promoter, at the optimal work temperature (60 °C) in the presence of 4 Å MS, different solvents and reaction vessels (Table 2). In the reactions carried out in a Schlenk tube, the starting materials were exclusively recovered (Table 2, entries 2–5) regardless of the solvent used, except for acetonitrile, which led to 8% conversion (Table 2, entry 6). Note that hexafluorobenzene, a solvent with a quadrupolar moment similar to CO2,26 did not cause any conversion (Table 2, entry 5). Microwave irradiation did not produce any glycosylated product either (Table 2, entry 7).
| Entry | Solvent | Time (h) | Container | Heating source | Conv.a (%) |
|---|---|---|---|---|---|
| a Conversion was determined by 1H NMR spectroscopy. b Glycosylation with galactosyl donor 5 (0.365 mmol, 1 equiv., 15 mM), glycosyl acceptor a (1.46 mmol, 4 equiv., 60 mM) and ca. 100 mg of 4 Å MS in a 25 mL stainless steel reactor (25 mL Parr reactor). c Reaction in 2 mL of solvent with glycosyl donor (0.03 mmol, 1 equiv., 15 mM), glycosyl acceptor (0.12 mmol, 4 equiv., 60 mM) and ca. 10 mg of 4 Å MS in a closed Schlenk tube. | |||||
| 1b | scCO2 (1500 Psi) | 20 | Reactor | Heat | >95 |
| 2c | Toluene | 20 | Schlenk tube | Heat | <1 |
| 3c | DCM | 20 | Schlenk tube | Heat | <1 |
| 4b | THF | 20 | Schlenk tube | Heat | <1 |
| 5c | C6F6 | 20 | Schlenk tube | Heat | <1 |
| 6c | CH3CN | 20 | Schlenk tube | Heat | 8 |
| 7c | Toluene | 2 | Microwave tube | Microwave irradiation | <1 |
| 8b | DCM (atm. P) | 20 | Reactor | Heat | <1 |
The fact that metallic traces coming from the inner wall of the stainless steel reactor might catalyse the glycosylation was also ruled out, since no conversion was observed when the reaction was run in CH2Cl2 at 60 °C and atmospheric pressure in the reactor (Table 2, entry 8).
All these results when compared with those obtained for the same reaction in scCO2 (Table 2, entry 1) enabled us to underline the unique role of scCO2, beyond an exclusive thermal effect.
Additional evidence for the activating role of scCO2 was obtained from a crossover experiment between tetra-O-pivaloyl-α-D-galactopyranosyl bromide (5) and tetra-O-acetyl-α-D-galactopyranosyl chloride (2), which were placed together in the reactor at 90 °C and 1500 Psi in the absence of a glycosyl acceptor (see the ESI† for experimental details). The crude mixture revealed the presence of three anomeric signals between 6.7 and 6.3 ppm, two of which corresponded to starting material 5 and the newly formed tetra-O-pivaloyl-α-D-galactopyranosyl chloride (6), thus accounting for a halogen exchange process. No traces of tetra-O-acetyl-α-D-galactopyranosyl bromide (1) were detected in the crude spectrum. Furthermore, the presence of a third anomeric signal might be due to the formation of a partially deacetylated galactosyl donor, although it was not identified.
The solubility of starting materials in the supercritical fluid is a key issue in this process. To shed some light on this regard we performed the reaction by physically separating the galactosyl donor from the acceptor within the reactor vessel. Thus, 2,3,4,6-tetra-O-pivaloyl-α-D-galactopyranosyl bromide (5) was introduced in a vial placed inside the reactor and the glycosyl acceptor (cyclohexanol) was introduced outside the vial in the same reactor. After 24 h at 60 °C and 1500 Psi (without stirring), NMR analysis of the final vial content showed the presence of some unreacted glycosyl donor with a large amount of product 9b. A similar situation was found outside the vial where no unreacted glycosyl acceptor but only product 9b was present. The experiment was repeated in the absence of the acceptor and introducing only the glycosyl donor 5 into the vial. After 24 h under supercritical conditions, compound 5 was uniformly distributed throughout the reactor.
The use of a reactor equipped with quartz windows led to an indisputable experimental piece of evidence for the solubility of the reactants under scCO2. Fig. 1 shows the change in the physical state of the starting material when subjected to scCO2 conditions. Thus, the mixture of glycosyl donor 5 and cyclohexanol appears as a homogeneous solution in scCO2 (Fig. 1c) whereas donor 5 alone (Fig. 1a) or the mixture of 5 and cyclohexanol (Fig. 1b) are displayed as heterogeneous under normal conditions.
Furthermore, the nature of the supercritical fluid was also evaluated (Table 3). Thus, the reaction of 2,3,4,6-tetra-O-pivaloyl-α-D-galactopyranosyl bromide 5 and cyclohexanol in Ar under the optimal conditions (1500 Psi, 60 °C) set for scCO2 proceeded with low conversion and preferential formation of the α anomer (Table 3, entry 2). In fact, the starting mixture appeared heterogeneous (see images in the ESI†). Moreover, when the reactants were physically separated inside the reactor vessel and the reaction was conducted in Ar, each starting material remained in its original location after careful depressurization. Although under these conditions, Ar is a supercritical fluid (Tc = 150.87 K, Pc = 48.26 atm = 710.39 Psi)27 it does not appear to dissolve the starting materials.
| Entry | Compressed gas | t (h) | Container | P | Conv.a (%) | α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β ratiob β ratiob |
|---|---|---|---|---|---|---|
a Conversion was determined by 1H NMR spectroscopy.
b The α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β ratio was determined by 1H NMR spectroscopy.
c Galactosyl donor (0.365 mmol, 1 equiv.), glycosyl acceptor (1.46 mmol, 4 equiv.) and ca. 100 mg of 4 Å MS in a 25 mL stainless steel reactor (25 mL Parr reactor).
d Galactosyl donor (0.365 mmol, 1 equiv.), glycosyl acceptor (1.46 mmol, 4 equiv.) and ca. 100 mg of 4 Å MS in a Schlenk tube. β ratio was determined by 1H NMR spectroscopy.
c Galactosyl donor (0.365 mmol, 1 equiv.), glycosyl acceptor (1.46 mmol, 4 equiv.) and ca. 100 mg of 4 Å MS in a 25 mL stainless steel reactor (25 mL Parr reactor).
d Galactosyl donor (0.365 mmol, 1 equiv.), glycosyl acceptor (1.46 mmol, 4 equiv.) and ca. 100 mg of 4 Å MS in a Schlenk tube.
|
||||||
| 1c | scCO2 | 24 | Reactor | 1500 Psi | >95 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 2c | scAr | 24 | Reactor | 1500 Psi | 25 | 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3c | CO2 | 24 | Reactor | 700 Psi | 36 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
| 4d | None | 24 | Schlenk | atm. P | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.1 10.1 |
| 5c | None | 24 | Reactor | atm. P | 40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
The reaction in scCO2 (Table 3, entry 1) proved far more superior to that run either in scAr (Table 3, entry 2) or subcritical CO2 (Table 3, entry 3), which proceeded with poor conversions and lower stereoselectivities. In addition, reactions under neat conditions led to low conversion (Table 3, entries 4 and 5), where the formation of the product could be accounted for the solvolysis of the galactosyl donor by the glycosyl acceptor.
During the preparation of this manuscript, a study by Leitner and Reetz was published, concluding that the use of scCO2 had no activating effect on alkylating reactions through the ionization of potentially SN1-active alkyl halides.28 Far from questioning the accuracy of their study, we believe that the differences between the systems studied by Leitner/Reetz and also by González-Nuñez (C–C bond formation in Friedel–Crafts and enol ether alkylation) and ours (C–O bond formation in a glycosylation reaction) could tell the difference. The acylated carbohydrates used as substrates in this study are much more complex substrates and present multiple basic sites, potentially able to interact with scCO2, thus enhancing possible clustering effects with respect to simple hydrocarbon substrates. Be as it may, we would like to present our contribution to the scientific community with the hope that accumulating experimental pieces of evidence will provide us more global knowledge about the nature and properties of scCO2.
Conclusions
The synthesis of O-glycosides in scCO2 has been developed, avoiding the use of VOCs as solvents and in the absence of transition metals as activators.The best results in terms of glycoside yield and stereoselectivity were obtained using pivaloyl-protected galactosyl bromides working in scCO2 at 60 °C and 1500 Psi. The scope of the reaction suggests that not only acetyl groups, but also benzoyl and pivaloyl groups can act as CO2-philic units.
The use of excess lutidine as an acid scavenger biased the reaction outcome towards the formation of the orthoester products in good yields, thus expanding the synthesis methodology for the preparation of this important type of glycosyl donor. Exclusive thermal glycosylation as well as an acid-promoted equilibration of glycoside products have been ruled out. The efficiency of scCO2 over scAr was also evidenced.
Although this study has focused on beta-glycosylation and the strongly solvent-dependent formation of alpha-glycosides may be anticipated to be more challenging, this reaction protocol is a proof of concept that glycosylation can become a green process.
Acknowledgements
We are indebted to Dr Anna M. Masdeu-Bultó (URV) for her kind disposition to share her pressure facilities with us. We gratefully acknowledge Prof. Walter Leitner at the RWTH Aachen University as well as Prof. M. Elena González-Nuñez at the Universitat de València for their helpful discussions. The financial support by the Ministerio de Economia y Competividad, Spain (CTQ2014-58664-R and RYC-2015-17705) and the European Regional Development Fund is gratefully acknowledged. A. C. thanks URV for a predoctoral fellowship. O. B. is a Ramón y Cajal Fellow.Notes and references
- L.-X. Wang and B. G. Davis, Chem. Sci., 2013, 4, 3381 RSC; C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357 CrossRef CAS PubMed; R. W. Dwek, Chem. Rev., 1996, 96, 683 CrossRef PubMed.
- M. C. Galán, D. Benito-Alifonso and G. M. Watt, Org. Biomol. Chem., 2011, 9, 3598 Search PubMed.
- M. A. Wolfert and G.-J. Boons, Nat. Chem. Biol., 2013, 9, 776 CrossRef CAS PubMed; M. Dalziel, M. Crispin, C. N. Scanlan, N. Zitzmann and R. A. Dwek, Science, 2014, 343, 37 CrossRef PubMed.
- A. Michael, Am. Chem. J., 1879, 1, 305 Search PubMed.
- E. Fischer, Ber. Dtsch. Chem. Ges., 1893, 26, 2400 CrossRef.
- B. Yu, J. Sun and X. Yang, Acc. Chem. Res., 2012, 45, 1227 CrossRef CAS PubMed; X. Zhu and R. R. Schmidt, Angew. Chem., Int. Ed., 2009, 48, 1900 CrossRef PubMed; A. V. Demchenko, Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance, Wiley-VCH, Weinheim, 2008 Search PubMed.
- K. Sasaki, D. Takahashi and K. Toshima, in Ionic liquids as Green Solvents for Glycosylation Reactions, Chapter 3 in Green Solvents II. Properties and applications of Ionic Liquids, ed. A. Mohammad and M. M. A. Inamuddin, Springer, Dordrecht, 2012 RSC. Glycosylation in ionic liquids and acid resin: F. J. Muñoz, S. André, H.-J. Gabius, J. V. Sinisterra, M. J. Hernáiz and R. J. Linhardt, Green Chem., 2009, 11, 373 RSC; C. Li, Z. Zhang, Q. Duan and X. Li, Org. Lett., 2014, 16, 3008 CrossRef CAS PubMed; I. J. Talisman, V. Kumar, J. Razzaghy and S. V. Malhotra, Carbohydr. Res., 2011, 346, 883 CrossRef PubMed; K. Prasad Sethi and K. P. R. Kartha, Carbohydr. Res., 2016, 434, 132 CrossRef PubMed; H. Nagai, K. Sasaki, S. Matsumura and K. Toshima, Carbohydr. Res., 2005, 340, 337 CrossRef PubMed; B. K. Gorityala, J. Ma, K. K. Pasunooti, S. Cai and X.-W. Liu, Green Chem., 2011, 13, 573 RSC; H. Komatsu and H. Umetani, Org. Process Res. Dev., 2002, 6, 847–850 CrossRef; R. S. Thombal and V. H. Jadhav, J. Carbohydr. Chem., 2016, 0, 1 Search PubMed; B. Roy and B. Mukhopadhyay, Tetrahedron Lett., 2007, 48, 3783 CrossRef; H. Hinou, N. Saito, T. Maeda, M. Matsuda, Y. Kamiya and S.-I. Nishimura, J. Carbohydr. Chem., 2011, 30, 575 CrossRef.
- Z. Wang, Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Hoboken, 2009, p. 1650 Search PubMed.
- D. Adam, Nature, 2000, 407, 938 CrossRef CAS PubMed.
- E. J. Beckman, J. Supercrit. Fluids, 2004, 28, 121 CrossRef CAS; W. Leitner, Nature, 2000, 405, 129 CrossRef PubMed; Handbook of Green Chemistry, Green Solvents, Supercritical Solvents, ed. W. Leitner and P. G. Jessop, Wiley-VCH, Weinheim, 2010 Search PubMed.
- E. Ramsey, Q. Sun, Z. Zhang, C. Zhang and W. Gou, J. Environ. Sci., 2009, 21, 720 CrossRef CAS.
- Examples of reactions in scCO2: synthesis of tetrasubstituted olefins via palladium catalysts: H.-F. Jiang, Q.-X. Xu and A.-Z. Wang, J. Supercrit. Fluids, 2009, 49, 377 CrossRef CAS . Asymmetric Diels–Alder reactions: S. Fukuzawa, K. Metoki and S. Esumi, Tetrahedron, 2003, 59, 10445 CrossRef . Olefin metathesis: M. Selva, A. Perosa, M. Fabris and P. Canton, Green Chem., 2009, 11, 229 RSC; F. Michalek, D. Mädge, J. Rühe and W. Bannwarth, Eur. J. Org. Chem., 2006, 577 CrossRef; A. Fürstner, L. Ackermann, K. Beck, H. Hori, D. Koch, K. Langemann, M. Liebl, Ch. Six and W. Leitner, J. Am. Chem. Soc., 2001, 123, 9000 CrossRef . Oxidation of alcohols: R. Ciriminna, P. Hesemann, J. J. E. Moreau, M. Carraro, S. Campestrini and M. Pagliaro, Chem. – Eur. J., 2006, 12, 5220 CrossRef PubMed; M. E. González-Núñez, R. Mello, A. Olmos, R. Acerete and G. Asensio, J. Org. Chem., 2006, 71, 1039 CrossRef PubMed . Formation of esters: M. Rezayat and H. S. Ghaziaskar, Green Chem., 2009, 11, 710 RSC . Friedel–Crafts alkylation: I. Komoto and S. Kobayashi, Org. Lett., 2002, 4, 1115 CrossRef PubMed . Aldol- and Mannich-type reactions: I. Komoto and S. Kobayashi, J. Org. Chem., 2004, 69, 680 CrossRef PubMed . The Baylis–Hillman reaction: P. M. Rose, A. A. Clifford and C. M. Rayner, Chem. Commun., 2002, 968 RSC . Nitroaldol condensations: R. Ballini, M. Noè, A. Perosa and M. Selva, J. Org. Chem., 2008, 73, 8520 CrossRef PubMed . Azide–alkyne cycloaddition: B. Grignard, S. Schmeits, R. Riva, C. Detrembleur, P. Lecomte and C. Jérôme, Green Chem., 2009, 11, 1525 RSC . Formation of cyclic carbonates: H.-F. Jiang, A.-Z. Wang, H.-L. Liu and C.-R. Qi, Eur. J. Org. Chem., 2008, 2309 CrossRef; Y. Kayaki, M. Yamamoto and T. Ikariya, J. Org. Chem., 2007, 72, 647 CrossRef PubMed; J.-Q. Wang, D.-L. Kong, J.-Y. Chen, F. Cai and L.-N. He, J. Mol. Catal. A: Chem., 2006, 249, 143 CrossRef . Carbamates and ureas: M. J. Fuchter, C. J. Smith, M. W. S. Tsang, A. Boyer, S. Saubern, J. H. Ryan and A. B. Holmes, Chem. Commun., 2008, 2152 RSC.
- P. Raveendran, Y. Ikushima and S. Wallen, Acc. Chem. Res., 2005, 38, 478 CrossRef CAS PubMed; E. J. Beckman, J. L. Reynolds, J. A. Gardecki, S. J. V. Frankland, M. L. Horng and M. Maroncelli, J. Phys. Chem., 1996, 100, 10337 CrossRef; J. F. Kauffman, J. Phys. Chem. A, 2001, 105, 3433 CrossRef.
- P. Raveendran and S. L. Wallen, J. Am. Chem. Soc., 2002, 124, 7274 CrossRef CAS PubMed.
- X.-B. Li, M. Ogawa, T. Monden, T. Maeda, E. Yamashita, M. Naka, M. Matsuda, H. Hinou and S.-I. Nishimura, Angew. Chem., Int. Ed., 2006, 45, 5652 CrossRef CAS PubMed; H. Hinou, N. Saito, M. Ogawa, T. Maeda and S.-I. Nishimura, Int. J. Mol. Sci., 2009, 10, 5285 CrossRef PubMed.
- T. Delgado-Abad, J. Martínez-Ferrer, A. Caballero, A. Olmos, R. Mello, M. E. González-Núñez, P. J. Pérez and G. Asensio, Angew. Chem., Int. Ed., 2013, 52, 13298 CrossRef CAS PubMed; T. Delgado-Abad, J. Martínez-Ferrer, J. Reig-López, R. Mello, R. Acerete, G. Asensio and M. E. González-Núñez, RSC Adv., 2014, 4, 51016 RSC.
- S.-G. Kazarian, M. F. Vincent, F. V. Bright, C. L. Liotta and C. A. Eckert, J. Am. Chem. Soc., 1996, 118, 1729 CrossRef CAS; J. F. Brennecke and J. E. Chateauneuf, Chem. Rev., 1999, 99, 433 CrossRef PubMed; M. Saharay and S. Balasubramanian, J. Phys. Chem. B, 2007, 111, 387 CrossRef PubMed.
- D. W. Arnold, S. E. Bradforth, E. H. Kim and D. M. Neumark, J. Chem. Phys., 1995, 102, 3493 CrossRef CAS.
- L. O. Kononov, N. N. Malysheva, A. V. Orlova, A. I. Zinin, T. V. Laptinskaya, E. G. Kononova and N. G. Kolotyrkina, Eur. J. Org. Chem., 2012, 1926 CrossRef CAS.
- Additionally, downward bias of the isolated yields due to the setup of the high-pressure equipment leading to small systematic mass loss could not be discarded.
- L. Bohe and D. Crich, Carbohydr. Res., 2015, 403, 48 CrossRef CAS PubMed.
- W. Pilgrim and P. V. Murphy, J. Org. Chem., 2010, 75, 6747 CrossRef CAS PubMed; C. O'Brien, M. Kolápová, N. Pitt, M. Tosin and P. V. Murphy, Chem. – Eur. J., 2007, 13, 902 CrossRef PubMed; G. Lanz and R. Madsen, Eur. J. Org. Chem., 2016, 3119 CrossRef.
- J. Lu and B. Fraser-Reid, Org. Lett., 2004, 6, 3051 CrossRef CAS PubMed; K. V. Radhakrishnan, V. S. Sajisha and J. M. Chacko, Synlett, 2005, 997 CrossRef.
- N. K. Kochetkov, A. J. Khorlin and A. F. Bochkov, Tetrahedron, 1967, 23, 693 CrossRef CAS; F. Kong, Carbohydr. Res., 2007, 342, 345 CrossRef PubMed; B. Fraser-Reid, S. Grimme, M. Piacenza, M. Mach and U. Schlueter, Chem. – Eur. J., 2003, 9, 4687 CrossRef PubMed; N. M. Podvalnyy, S. L. Sedinkin, P. I. Abronina, A. I. Zinin, K. G. Fedina, V. I. Torgov and L. O. Kononov, Carbohydr. Res., 2011, 346, 7 CrossRef PubMed; G. Sureshkumar and S. Hotha, Tetrahedron Lett., 2007, 48, 6564 CrossRef.
- A. Padwa, J. D. Ginn, S. K. Bur, C. K. Eidell and S. M. Lynch, J. Org. Chem., 2002, 67, 3412 CrossRef CAS PubMed; H. P. Wessel and N. Ruiz, J. Carbohydr. Chem., 1991, 10, 901 CrossRef.
- J. Vrbancich and G. L. Ritchie, J. Chem. Soc., Faraday Trans. 2, 1980, 648 RSC.
- M. W. Haynes, Handbook of Chemistry and Physics, CRC Press, Boca Raton, Florida, USA, 92nd edn, 2011, p. 4121 Search PubMed.
- Y. X. Qiao, N. Theyssen, T. Eifert, M. A. Liauw, G. Franciò, K. Schenk, W. Leitner and M. T. Reetz, Chem. – Eur. J., 2017, 23, 3898 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7gc00722a |
| This journal is © The Royal Society of Chemistry 2017 |