Open Access Article
Open Access ArticleGreener solvents for solid-phase synthesis†‡
Stefan
Lawrenson
 ,
Michael
North
,
Michael
North
 *,
Fanny
Peigneguy
and
Anne
Routledge
*,
Fanny
Peigneguy
and
Anne
Routledge
 *
*
Department of Chemistry, University of York, York, Y10 5DD, UK. E-mail: michael.north@york.ac.uk; anne.routledge@york.ac.uk
First published on 21st December 2016
Abstract
The use of a variety of green solvents to swell a diverse range of resins used in solid-phase synthesis is investigated. Good swelling is shown to depend on the structure of the resin and the solvent. A modelling approach based on use of a training set of solvents is used to predict which green solvents will, and will not, swell a particular resin. The chemical relevance of the swelling results is confirmed by an experimental study of a solid-supported Ugi reaction carried out in green solvents.
Introduction
For much of the chemicals industry, solvent is the largest source of waste associated with chemical production. This is particularly apparent in the high E factors1 associated with the fine chemicals and pharmaceuticals sectors where solvent typically accounts for 80–90% of the total mass utilisation in batch operations.2 Some widely used solvents such as DMF, DMA and NMP are known to be reprotoxic3 and have therefore been classified as three of the six substances of highest concern under the REACh regulations.4 As a result they are a high priority for replacement by greener solvents. Other widely used solvents such as dichloromethane and diethyl ether have low boiling points leading to potentially high VOC emissions and low flash points.As a result, the pharmaceutical sector in particular has taken the lead in investigating the replacement of conventional solvents and this has resulted in the publication of a number of green solvent guides5,6 and a solvent life cycle analysis study.7 However, these guides have focussed on conventional solution-phase synthesis where the main roles of the solvent are: to dissolve reactants, intermediates and products, to facilitate stirring of the reaction components and to facilitate heat transfer in or out of the reaction vessel. Another approach to chemical production is solid-phase synthesis in which either one of the reactants (and hence the product) is immobilised on a solid-support, or one or more reagents are immobilised with the product remaining in solution. The former approach is exemplified by solid-phase peptide synthesis (SPPS),8 whilst the widely used and commercially available cross-linked polystyrene supported triphenylphosphine9 illustrates the second approach. Solid-phase synthesis imposes an additional requirement on the solvent: it must swell the resin to provide access to reactive functionalities which would otherwise be blocked within the polymer matrix. The use of green solvents in solid-phase synthesis has not previously been systematically investigated, so we undertook a study based around the ability of green solvents to swell resins commonly used in solid-phase synthesis and in this paper report the results of this work.
Results and discussion
Resin swelling studies
For this study, nine commercially available resins for solid-phase synthesis were selected (Table 1). Merrifield,10 ParaMax11 and JandaJel™![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 are all polystyrene based resins but with different functionalities and cross-linkers. Merrifield resin is polystyrene crosslinked with 1,4-divinybenzene originally developed by Merrifield for SPPS. For this study it was functionalised with a Wang linker13 (4-hydroxybenzyl alcohol). ParaMax is similar to Merrifield resin but with the functionalisation (hydroxymethyl for this study) exclusively in the para-positions of the aromatic rings. JandaJel™ has a more flexible, butane-based cross-linker than that present in Merrifield and ParaMax resins.
12 are all polystyrene based resins but with different functionalities and cross-linkers. Merrifield resin is polystyrene crosslinked with 1,4-divinybenzene originally developed by Merrifield for SPPS. For this study it was functionalised with a Wang linker13 (4-hydroxybenzyl alcohol). ParaMax is similar to Merrifield resin but with the functionalisation (hydroxymethyl for this study) exclusively in the para-positions of the aromatic rings. JandaJel™ has a more flexible, butane-based cross-linker than that present in Merrifield and ParaMax resins.
| Resin name | Polymer backbone | Functionality | Bead size (mesh) | Capacity (mmol g−1) |
|---|---|---|---|---|
| Merrifield | 1% crosslinked polystyrene | Wang (ArCH2OH) | 200–400 | 0.6–1.0 |
| ParaMax | 1% crosslinked polystyrene | CH2OH in para positions | 100–200 | 2.0 |
| JandaJel | Polystrene crosslinked with 2% 1,4-di(4-vinylphenoxy)butane | CH2Cl | 100–200 | 0.8–1.2 |
| TentaGel S RAM Fmoc | Polyethylene glycol grafted onto 1% crosslinked polystyrene | Fmoc protected Rink amide | 80–100 | 0.23 |
| ArgoGel | Polyethylene glycol grafted onto polystyrene. Higher PEG than Tentagel | Cl | 200–400 | 0.48 |
| HypoGel 200 | Polyethylene glycol grafted onto polystyrene. Shorter PEG than Tentagel | CO2H | 110–150 | 0.8 |
| NovaGel | Polyethylene glycol grafted onto polystyrene | HMPA (ArCH2OH) | Not stated | 0.74 |
| ChemMatrix | Crosslinked PEG resin | Wang (ArCH2OH) | 35–100 | 0.5–1.2 |
| SpheriTide | Crosslinked lysine | Fmoc protected Rink amide | 45–140 | 0.21 |
The next set of four resins all had structures consisting of polyethylene glycol (PEG) grafted onto cross-linked polystyrene. TentaGel™![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 is around 70% PEG by weight with each PEG chain having an average molecular weight of 3000 Daltons. It was functionalised with a 9-fluorenylmethoxycarbonyl (Fmoc) protected Rink15 amide linker. ArgoGel™
14 is around 70% PEG by weight with each PEG chain having an average molecular weight of 3000 Daltons. It was functionalised with a 9-fluorenylmethoxycarbonyl (Fmoc) protected Rink15 amide linker. ArgoGel™![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 is similar to TentaGel but two PEG chains are grafted onto each aromatic ring. This gives it a higher capacity and it consists of up to 82% PEG. For this study, the PEG chains were terminated with chloro groups to provide a contrasting functionality to the Tentagel. HypoGel™ 20017 in contrast has PEG chains that are just five ethylene glycol units long and these were terminated with carboxylic acid groups. Finally, NovaGel™ is a high swelling resin in which the PEG groups are attached to the polystyrene core by urethane groups.18 It consists of 48% PEG and was functionalised with (4-hydroxymethylphenyl)acetic acid (HMPA) to provide alcohol functionalities for substrate immobilisation.
16 is similar to TentaGel but two PEG chains are grafted onto each aromatic ring. This gives it a higher capacity and it consists of up to 82% PEG. For this study, the PEG chains were terminated with chloro groups to provide a contrasting functionality to the Tentagel. HypoGel™ 20017 in contrast has PEG chains that are just five ethylene glycol units long and these were terminated with carboxylic acid groups. Finally, NovaGel™ is a high swelling resin in which the PEG groups are attached to the polystyrene core by urethane groups.18 It consists of 48% PEG and was functionalised with (4-hydroxymethylphenyl)acetic acid (HMPA) to provide alcohol functionalities for substrate immobilisation.
The final two resins were chosen to contain no polystyrene. ChemMatrix19 is a crosslinked PEG that is known to swell well in a wide range of solvents. It was functionalised with a Wang linker13 to provide alcohol functionalities. SpheriTide is a polyamide resin comprising crosslinked lysine units.20 It was used with an Fmoc-protected Rink linker.15
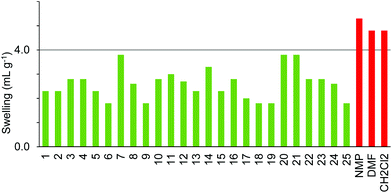
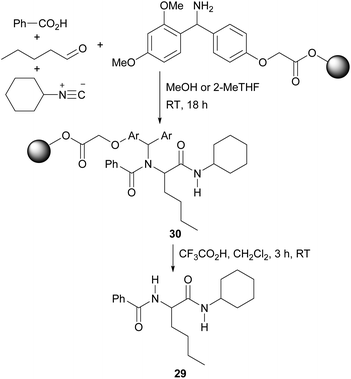
The swelling of each resin in solvents was determined using the method of Griffith et al.,21 measuring the increase in volume occupied by a resin sample held in a syringe on addition of the appropriate solvent. A solvent in which a resin swells to greater than 4.0 mL g−1 is considered a good solvent, 2.0–4.0 mL g−1 a moderate solvent and less than 2.0 mL g−1 a poor solvent.21 Initial studies using Merrifield-Wang resin in three conventional solvents (DMF, NMP and dichloromethane, which are known to give good resin swelling) showed a good correlation with literature data21 (Fig. 1).
 | ||
| Fig. 1 Swelling of Merrifield resin in three conventional solvents. Red: swelling measured in this work; black: literature values using the same methodology.21 | ||
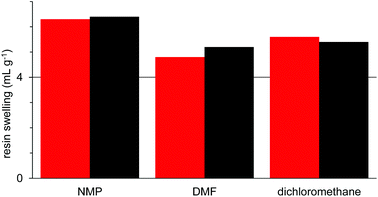
Having validated the methodology, 25 green solvents with a wide range of polarities and hydrogen bond donor/acceptor properties were selected as potential green solvents for solid-phase synthesis (Fig. 2). Cyclic carbonates221 and 2 and Cyrene233 have all been used as green replacements for traditional polar aprotic solvents. The cyclic carbonates can be prepared by the 100% atom economical reaction between epoxides and carbon dioxide,24,25 whilst Cyrene is prepared from glucose by pyrolysis followed by hydrogenation.23
 | ||
| Fig. 2 Structures of the green solvents used in this study (data in brackets is the boiling point at atmospheric pressure). | ||
The ethers, ketones, esters and acyclic carbonates 4–17 can all be considered moderately polar aprotic solvents. This group contains two well-known conventional solvents (acetone 4 and ethyl acetate 8) which have reasonable green credentials. γ-Valerolactone2611 and 2-methyl-THF2712 are both bioderived from sugars, whilst acyclic carbonates 16 and 17 can be prepared from carbon dioxide and sustainably sourced alcohols.28 Cyclopentanone 7, isobutyl acetate 10 and anisole 13 were included as they scored very highly in the latest version of the GSK green solvents guide.5c Dimethyl isosorbide 14, cyclopentyl methyl ether 15, butan-2-one 5, 4-methylpentan-2-one 6 and isopropyl acetate 9 were included as a result of the modelling studies reported later in this paper. D-Limonene 18 is a natural product and can readily be converted into para-cymene 19.29 Therefore compounds 18 and 19 represent non-polar aliphatic and aromatic solvents respectively. The set of protic solvents chosen includes three conventional alcoholic solvents (20–22), all of which can be obtained from sustainable sources,30 1-heptanol 23 which scored highly in the latest version of the GSK green solvents guide5c and water 24.31 Finally, ionic liquid 25 was included as an example of a non-conventional solvent.
The swelling of the nine resins detailed in Table 1 was investigated in each of solvents 1–25 as well as in DMF, NMP and dichloromethane for comparison and the results are shown in Fig. 3–11. Three of the resins (Merrifield, ArgoGel and ChemMatrix) and 8 solvents (2, 4, 8, 12, 17, 20, 24 and dichloromethane) were selected to check the reproducibility of the data shown in Fig. 3–11. The swelling of all 24 combinations of these resins and solvents was measured five times and in all cases the results were reproducible to within ±0.5 mL g−1.32
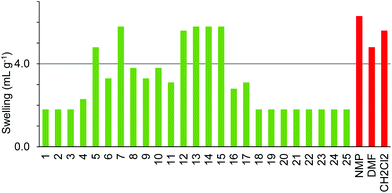
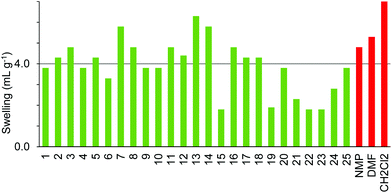
Comparison of the data in Fig. 3–5 shows differences in swelling behaviour between the three polystyrene-based resins. Thus, Merrifield resin (Fig. 3) only swells well in the moderately polar, oxygenated solvents; butan-2-one 5, cyclopentanone 7, 2-methyl-THF 12, anisole 13, dimethyl isosorbide 14 and cyclopentyl methyl ether 15. In contrast, ParaMax (Fig. 4) was found to show better swelling in a range of solvents than Merrifield resin and swells well in all the moderately polar, oxygenated solvents 4–18. JandaJel (Fig. 5), which has a more flexible crosslinker than Merrifield and ParaMax resins was found to swell well in moderately polar, oxygenated solvents 5–15 (but not in acetone 4 or acyclic carbonates 16 and 17) and also swelled in the two very non-polar solvents; limonene 18 and para-cymene 19 which did not swell Merrifield or ParaMax resins. All of the polar aprotic solvents (1–3), protic solvents (20–24) and ionic liquid 25 were found to be poor solvents to induce swelling in any of the three aromatic resins.
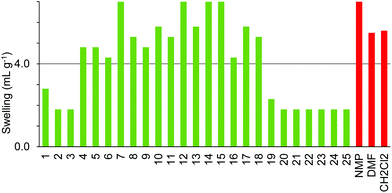
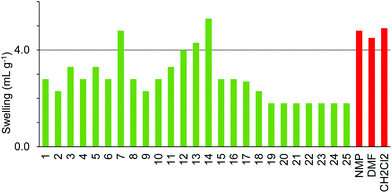
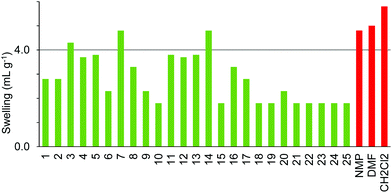
The four resins composed of PEG grafted onto polystyrene (TentaGel S, ArgoGel, HypoGel 200 and NovaGel) gave the results shown in Fig. 6–9. TentaGel S (Fig. 6) was found to only swell to more than 4.0 mL g−1 in cyclopentanone 7 and dimethyl isosorbide 14 though most of the other polar and moderately polar solvents swelled the resin to just below 4.0 mL g−1. ArgoGel (Fig. 7) which has more PEG character than TentaGel S was found to be a very good resin for use with green solvents, swelling well in polar aprotic (2, 3), moderately polar oxygenated (5, 7, 8, 11–14 and 16, 17) and even non-polar (18) solvents. Four other oxygenated aprotic solvents (1, 4, 9 and 10) swelled the resin to just below 4.0 mL g−1 as did methanol (20) and ionic liquid 25, though cyclopentyl methyl ether 15 was a very poor solvent for this resin. HypoGel 200 (Fig. 8) and NovaGel (Fig. 9) gave similar results to TentaGel S, though 2-methyl-THF 12 and anisole 13 gave good swelling of HypoGel 200 whilst they swelled TentaGel S to just below 4.0 mL g−1. For NovaGel, the polar aprotic solvent, Cyrene 3, swelled the resin to over 4.0 mL g−1 whilst it swelled TentaGel S to just below 4.0 mL g−1. Notably, TentaGel S, HypoGel 200 and NovaGel were functionalised in three different ways (Fmoc-protected amine, carboxylic acid and benzylic alcohol) yet had very similar swelling properties in the green solvents which suggests that the observed variation in swelling with solvent is a function of the resin backbone and not of the way in which it is functionalised. This is important for synthetic applications as the nature of the functionality attached to the resin will change during a solid-phase synthesis.
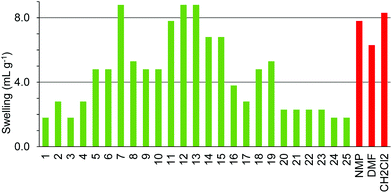
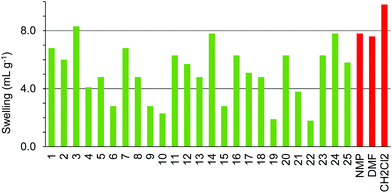
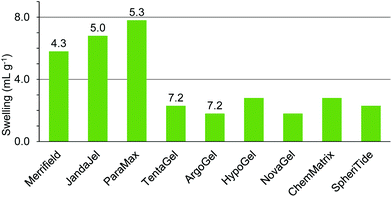
ChemMatrix resin was found to swell well in a wide range of green solvents (Fig. 10). The only solvents found not to be suitable for use with this resin were 4-methylpentan-2-one 6, isopropyl acetate 9, isobutyl acetate 10, cyclopentyl methyl ether 15, para-cymene 19, ethanol 21 and isopropanol 22. The incompatibility of this resin with aromatic solvent 19 is understandable as the resin contains no aromatic groups. The lack of swelling in the more non-polar ketones and esters 6, 9, 10 when very similar but more polar ketones 4, 5 and ester 8 did swell the resin well probably reflects the polar nature of the PEG-based polymer. Curiously however, whilst ethanol 21 and isopropanol 22 failed to swell the resin, the more polar alcohol; methanol 20 and the less polar alcohol 1-heptanol 23 both gave good swelling of the resin.
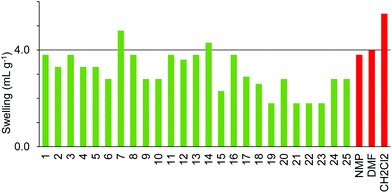
The SpheriTide resin did not swell well in any of the green solvents (Fig. 11), though cyclopentanone 7 and the more polar alcohols; methanol 20 and ethanol 21 did swell it to just below 4.0 mL g−1. The SpheriTide resin was functionalised with an Fmoc-protected Rink linker, exactly the same functionality present on the TentaGel S resin. Comparison of Fig. 6 and 11 shows very different swelling behaviour for these two resins across a range of green solvents, again suggesting that interactions between the solvent and resin determine the resin swelling, not the nature of the functionalisation.
Comparison of the data in Fig. 3–10 shows that cyclopentanone 7 and dimethyl isosorbide 14 were able to swell all of the polystyrene and PEG based resins to more than 4.0 mL g−1. In contrast, ethanol 21 and isopropanol 22 were not able to swell any of the resins to 4.0 mL g−1 and ionic liquid 25 was only suitable for use with ChemMatrix resin. The ChemMatrix and ArgoGel resins appear to have the best overall compatibility with a range of green solvents.
Modelling resin–solvent interactions
To better understand and be able to predict resin swelling in green solvents, a modelling study was undertaken using the Hansen Solubility Parameters in Practice (HSPiP) software.33A training set of 15 green solvents (1–4, 7, 8, 11, 12, 16–22) as well as DMF, NMP and dichloromethane was chosen. For each resin, these solvents were divided into n groups (n = 4–6) based on their ability to swell the resin. Thus, the difference between the maximum (Smax) and minimum (Smin) swelling volume observed for a given resin with these 18 solvents was divided by n and the solvents allocated to groups32 given by:
| Group ‘a’ lower boundary = Smax − a((Smax − Smin)/n) |
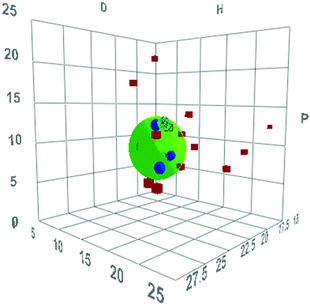
The software then analyses solvents in terms of their ability to swell the resin and carries out a least squares minimisation to produce a three-dimensional plot based on the dispersion energies (D), hydrogen bonding energies (H) and dipolar energies (P) of the solvents such as that shown in Fig. 12 for Merrifield resin with n = 5 (plots for all resins with n = 4, 5 and 6 are given in the ESI‡). In this plot, the centre of the green sphere is at the parameters of the ideal solvent to swell the resin and the radius of the sphere (R) represents the uncertainty in this value. The blue circles are the group 1 (highest swelling) solvents and the red squares are all the other solvents in the training set.
Table 2 shows the numerical results obtained for all nine resins with four, five or six groups. The HSPiP software requires there to be two solvents in the highest swelling group (group 1). If one solvent swells the resin much better than any other, then the default software parameters will not be able to calculate the optimal solvent parameters. This was found to be the case for ArgoGel where dichloromethane produced much greater swelling than any other solvent. In addition, as the value of n increases, it becomes increasingly likely that there will be only one solvent in the highest swelling group and when n = 6 this was found to be the case for TentaGel, NovaGel and ChemMatrix resins. Both these problems could be solved within the software by giving equal weighting to solvents in groups 1 and 2 when required.
| Resin | n = 4 | n = 5 | n = 6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | P | H | R | D | P | H | R | D | P | H | R | |
| a Value calculated giving equal weighting to solvents in groups 1 and 2. | ||||||||||||
| Merrifield | 17.48 | 8.51 | 4.27 | 3.3 | 17.47 | 8.27 | 4.77 | 4.9 | 17.65 | 11.24 | 9.43 | 3.0 |
| ParaMax | 17.68 | 7.87 | 7.71 | 4.0 | 17.68 | 7.87 | 7.72 | 4.0 | 17.68 | 7.82 | 7.75 | 4.0 |
| JandaJel | 17.13 | 9.34 | 4.09 | 3.4 | 17.30 | 9.16 | 4.09 | 3.4 | 16.97 | 10.23 | 2.48 | 5.5 |
| TentaGel S RAM Fmoc | 17.76 | 10.88 | 6.49 | 1.7 | 17.72 | 10.82 | 6.41 | 1.7 | 17.69a | 10.78a | 6.35a | 1.7a |
| ArgoGel | 18.37a | 8.44a | 8.44a | 3.3a | 17.68a | 10.93a | 6.40a | 1.7a | 17.71a | 10.81a | 6.40a | 1.7a |
| HypoGel 200 | 17.33 | 11.72 | 9.05 | 4.9 | 17.57 | 12.02 | 8.68 | 3.9 | 17.78 | 12.27 | 9.10 | 4.9 |
| NovaGel | 17.78 | 11.37 | 9.57 | 4.7 | 17.21 | 10.87 | 10.22 | 3.0 | 17.90a | 12.10a | 9.07a | 4.9a |
| ChemMatrix | 18.58 | 9.89 | 7.04 | 1.0 | 18.60 | 9.82 | 6.98 | 1.0 | 17.80a | 11.20a | 9.27a | 3.2a |
| SpheriTide | 17.45 | 11.10 | 9.61 | 3.0 | 17.14 | 11.33 | 9.35 | 3.0 | 17.26 | 11.24 | 9.43 | 3.0 |
Based on the results in Table 2, n = 5 gives the optimal results in terms of having the lowest value for R (in all cases except Merrifield resin) and values for D P and H which are close to those for n = 4 and 6. The parameters calculated for the optimal solvents to swell polystyrene and PEG resins could be compared with the D, P and H parameters for ethylbenzene (D = 17.8, P = 0.6, H, 1.4) and ethylene glycol dimethyl ether (D = 15.4, P = 6.3, H, 6.0) as models for the monomer units within the two polymers. For polystyrene resins, the dispersion energies (D) of the model monomer unit and predicted optimal solvents matched quite well (17.1–17.7 to 17.8), but the dipolar energies (P) and hydrogen bonding energies (H) were very different. For the PEG-based resin, ChemMatrix, none of the energy parameters was a particularly good fit. This indicates that it is not possible to assume ‘like swells like’ and just base the modelling on the polymer repeat unit.
Having determined the energy parameters for an optimal solvent for each resin, the HSPiP software then generated a list of all solvents in its database ranked in order of their least squares distance from the optimal solvent.32 The solvents (excluding those in the training set) in these tables were coded green amber or red according to the latest version of the GSK green solvents guide5c and Table 3 lists those green or amber solvents that were found to swell at least 4 resins.
| Resin | Solventa | ||||||
|---|---|---|---|---|---|---|---|
| 5 | 6 | 9 | 14 | 15 | 26 | 27 | |
| a The number given for each solvent is its RMS deviation from the DHP parameters for an optimal solvent for the resin. | |||||||
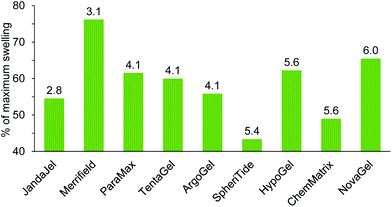
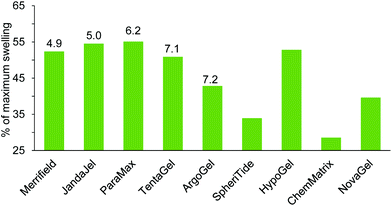
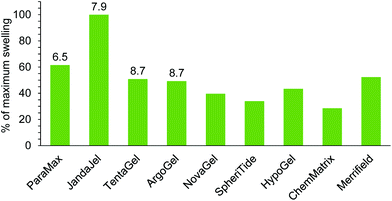
| Merrifield | 3.1 | 4.9 | 3.0 | 4.3 | |||
| ParaMax | 4.4 | 6.2 | 6.5 | 0.8 | 5.3 | 4.3 | |
| JandaJel | 2.8 | 5.0 | 7.9 | 4.0 | 5.0 | 7.0 | |
| TentaGel | 4.1 | 7.1 | 8.7 | 3.9 | 7.2 | 7.3 | 8.5 |
| ArgoGel | 4.1 | 7.2 | 8.4 | 4.0 | 7.2 | 7.4 | 8.5 |
| HypoGel 200 | 5.6 | 5.1 | 6.8 | ||||
| NovaGel | 6.0 | 4.7 | 6.6 | 4.7 | |||
| ChemMatrix | 5.6 | 3.4 | |||||
| SpheriTide | 5.4 | 4.7 | 5.6 | ||||
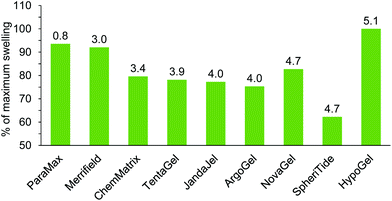
Glycerol triacetate 26 was found to be a very viscous solvent and this prevented experimental determination of its ability to swell resins. Glycerol diacetate 27 is only available as a mixture of 1,2- and 1,3-regioisomers, so it was also omitted from experimental studies. The remaining solvents in Table 3, (5, 6, 9, 14 and 15) were included in the solvents used in the experimental determination of resin swelling (Fig. 3–11) which allowed the accuracy of the solvent selection predictions to be tested and the experimental and predicted swellings for each resin are shown in Fig. 13–17. The data in Fig. 13–17 is presented as a percentage of the maximum observed swelling for that resin to compensate for the fact that some resins swell more than others and so allow data to be compared between resins.
It is apparent from Fig. 13 that there is a good correlation between how close the DHP parameters for dimethyl isosorbide 14 are to the DHP parameters for the ideal solvent for the resin and how much the resin was experimentally found to swell in solvent 14. The only exceptions are NovaGel for which the experimental swelling was 10–20% higher than predicted and HypoGel. In the latter case, dimethyl isosorbide 14 was found to be a better solvent to swell the resin than any solvent in the training set or in Fig. 2. This is an indication that the swelling of HypoGel by dimethyl isosorbide is influenced by one or more parameters not covered by solvents in the training set. Of the nine resins (all of which were predicted to swell in solvent 14 on the basis of the data in Table 3), dimethyl isosorbide 14 was experimentally found to swell all but SpheriTide to more than 4.0 mL g−1 (Fig. 3–11 and ESI‡).
A similar situation is apparent in Fig. 14 for the swelling of resins with cyclopentyl methyl ether 15. In this case solvent 15 was found to be the equal best solvent (with NMP) to swell paraMax and the experimental result is about 30% higher than predicted by the modelling study. Otherwise, the experimentally observed swelling of the first five solvents decreases as the RMS distance of the DHP parameters for cyclopentyl methyl ether 15 from those of the ideal solvent increases. For this solvent, only Merrifield, JandaJel and ParaMax resins were found to swell to more than 4.0 mL g−1 (Fig. 3–11 and ESI‡).
The data for resin swelling in butan-2-one 5 (Fig. 15) is more scattered, although a general decrease in the percentage of maximum resin swelling as the RMS distance of the DHP parameters for butan-2-one 5 from those of the ideal solvent increases is apparent. In Fig. 15, the swelling of JandaJel appears to be too low and the swelling of HypoGel and NovaGel too high. However, this is largely a feature of the swelling of these resins in other solvents affecting the results when displayed as a percentage. There was actually very little difference in the range of resin swellings observed in butan-2-one (2.3–4.8 mL g−1)32 which is probably largely responsible for the scatter seen in Fig. 15. Nevertheless, of the five resins with the lowest RMS deviations for butan-2-one 5, four were found to swell to >4.0 mL g−1.32
For 4-methylpentan-2-one 6 (Fig. 16) and isopropyl acetate 9 (Fig. 17), the RMS distance of the DHP parameters in all of the solvents were rather large (>4.8). Nevertheless, a general decrease in percentage of maximum resin swelling as the RMS value increased is apparent, though the swelling of JandaJel in isopropyl acetate appears to be higher than predicted. For both solvents, only two resins were found to swell to >4.0 mL g−1 and these were JandJel and ParaMax which were ranked second and third in 4-methylpentan-2-one and first and second in isopropyl acetate.32
Overall the data in Fig. 13–17 shows that the modelling approach adopted using the HSPiP software can be used to suggest new green solvents to swell a particular resin.
To further investigate the applicability of the HSPiP software to green solvent prediction, its ability to predict mixed solvent systems capable of swelling resins was investigated. The solvent optimization list within HSPiP was edited to contain just solvents 1–24 and this was used in conjunction with the (n = 5) ideal D, P and H parameters for each resin (Table 2) to generate an optimal mixture of two solvents to swell each resin. The results are shown in Table 4.
| Resin | Predicted | Experimental | |||
|---|---|---|---|---|---|
| Solvent 1 (%) | Solvent 2 (%) | Solvent 1 (mL g−1) | Solvent 2 (mL g−1) | Mixture (mL g−1) | |
| Merrifield | 12 (52) | 15 (48) | 5.6 | 5.8 | 4.8 |
| ParaMax | 14 (95) | 1 (5) | 7.3 | 2.8 | 7.8 |
| JandaJel | 12 (75) | 1 (25) | 8.8 | 1.8 | 8.3 |
| TentaGel | 7 (84) | 23 (16) | 4.8 | 1.8 | 3.8 |
| ArgoGel | 7 (84) | 23 (16) | 5.8 | 1.8 | 5.8 |
| HypoGel 200 | 7 (91) | 24 (9) | 4.8 | 1.8 | 5.3 |
| NovaGel | 7 (65) | 21 (45) | 4.8 | 1.8 | 4.3 |
| ChemMatrix | 3 (80) | 14 (20) | 8.3 | 7.8 | 8.8 |
| SpheriTide | 7 (71) | 21 (29) | 3.8 | 3.8 | 3.8 |
In five cases (JandaJel, TentaGel, ArgoGel, NovaGel, SpheriTide), the optimal predicted solvent mixture was found experimentally to swell the resin to an amount that was between or the same as the swelling observed with the solvent components individually. This is the result that would be expected in the absence of any cooperative effects between the two solvents. However, in three cases (ParaMax, HypoGel 200, ChemMatrix) the predicted solvent mixture was found to swell the resin more than either solvent individually and in one case (Merrifield), the predicted optimal solvent was found to swell the resin less than either solvent individually. These results could not have been predicted just on the basis of the swelling observed by the solvent components alone and are suggestive of positive or negative effects involving both solvents and their interaction with the resin. These results illustrate the ability of the HSPiP software to make predictions concerning resin swelling which are not intuitively obvious.
Resin-supported Ugi reactions
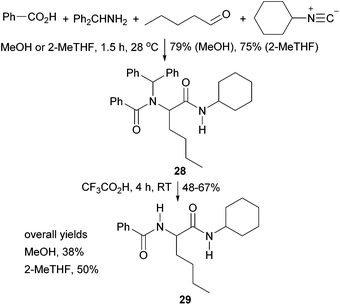
To experimentally test the relevance of the resin swelling results, we chose the Ugi reaction as a pharmaceutically relevant, 4-component condensation reaction which produces α-amino amides. The solution-phase Ugi reaction is known to occur in a wide range of solvents and solid-phase variants have been reported on a range of resins.34Two resins, Merrifield and ChemMatrix were selected along with two green solvents: methanol 20 and 2-methyl-THF 12. Methanol was chosen over other potentially green alcohols as it has been reported34b to be preferred solvent for solution phase Ugi reactions due to its polarity giving a high solubility of reagents, but low solubility of Ugi products. Based on the resin swelling results shown in Fig. 3 and 10, it was predicted that for a Merrifield resin-supported Ugi reaction, 2-methyl-THF would be a much better solvent than methanol whilst for a ChemMatrix supported reaction, both solvents would be effective. Initially, the solution-phase Ugi reaction (Scheme 1) was carried out in both solvents to test the methodology and to obtain baseline yields for the Ugi reactions in the two solvents in the absence of any resin swelling effects. Benzhydrylamine was chosen as the amine component to mimic the Rink amide linker which would be used for solid-supported reactions. The Ugi reactions in methanol and 2-methyl-THF proceeded in nearly identical yields (79 and 75% respectively after 1.5 hours), giving α-amino amide 28. Removal of the benzhydryl group was also carried out as this would correspond to cleavage of Ugi adducts from a solid-support and gave α-amino amide 29 in 48–67% yield. Thus, the overall yield for the synthesis of compound 29 in solution was 38% in methanol and 50% in 2-methyl-THF.
To convert this Ugi reaction to a solid-supported synthesis, the benzhydrylamine was replaced by Merrifield or ChemMatrix resin with a Rink amide linker attached (Scheme 2). Commercial resins supplied with Fmoc-protected Rink linkers were first deprotected with 20% piperidine in DMF. Then, the Ugi reactions were carried out as for the solution-phase synthesis of α-amino amide 28. The resin-supported α-amino amide 30 was cleaved from the support by treatment with trifluoroacetic acid in dichloromethane. DMF and dichloromethane were used for the initial resin Fmoc-deprotection and substrate cleavage steps as these are high swelling solvents for both resins (Fig. 3 and 10) thus ensuring that the isolated yields of α-amino amide 29 were representative of the Ugi reactions and not influenced by the resin cleavage. Compound 29 was then purified by flash chromatography to give isolated yields. All solid-supported syntheses were carried out in duplicate to ensure that the results were reproducible and the results are shown in Table 5.
| Resin name | Capacitya (mmol g−1) | Solvent | 29 (%) |
|---|---|---|---|
| a Obtained from the manufacturer's certificate of analysis and checked by CHN analysis. b Isolated yields after purification by column chromatography from two separate reactions. | |||
| Merrifield | 1.0 | 2-MeTHF | 27 and 36 |
| Merrifield | 1.0 | MeOH | 3 and 4 |
| ChemMatrix | 0.49 | 2-MeTHF | 52 and 57 |
| ChemMatrix | 0.49 | MeOH | 30 and 32 |
It is apparent from the data in Table 5, that for reactions carried out on Merrifield resin, the average yield obtained from reactions carried out in 2-methyl-THF 12 is about four times higher than the average yield obtained in methanol 20. This is consistent with 2-methyl-THF effectively swelling the resin and allowing reagents to access all of the resin-supported amines whilst methanol did not effectively swell the resin and only surface exposed amines were able to react. In contrast, for reactions carried out on ChemMatrix resin, the average yields obtained with both solvents very closely match the corresponding overall yields obtained from the solution phase synthesis of amide 29 (Scheme 1), which indicates that both solvents are able to effectively swell the ChemMatrix resin. Thus, the results obtained from the solid-supported Ugi reactions indicate that the experimentally determined resin swellings are indicative of which green solvents will (and will not) be suitable for solid-supported synthesis on a particular resin.
Experimental
General methods
All experiments were carried out under a precautionary atmosphere of N2. Analytical TLC was performed on aluminium backed plates pre-coated (0.25 mm) with Merck KGaA silica Gel 60 F254. Compounds were visualised by exposure to UV light and stained using potassium permanganate solution (KMnO4) followed by heating. Flash column chromatography was performed using Flurochem Silica Gel LC60A (40–60 μM). All solvent mixtures are reported as v/v solutions.1H- and 13C-NMR spectra were obtained using a JEOL ECS 400 MHz Spectrometer. Both 1H and 13C spectra were referenced to the residual protic solvent (CHCl3 = 7.26 ppm, CH3OH = 4.78 ppm, (CH3)2SO = 3.30). Coupling constants are reported using the following notation or combination of: s = singlet, br = broad, d = doublet, t = triplet, m = multiplet. Assignment of signals in 1H spectra was achieved using 1H–1H COSY spectra, where necessary.
High resolution mass spectra (HRMS) were recorded using Electrospray Ionisation (ESI), on a Bruker micrOTOF Mass Spectroscopy (MS) in tandem with an Agilent series 1200 liquid chromatography (LC) system. Infra-Red (IR) spectra were recorded using a Perkin Elmer Spectrum Two FT-IR spectrometer. Melting points were determined with a TA Instruments Q2000 DSC. Solvent modelling was carried out using HSPiP software.33
Determination of resin swelling
Resin (100 mg) was accurately weighed into a 2 mL syringe fitted with a polypropylene fritted disc (void volume = 0.12 mL). Solvent (2 mL) was added and the syringe agitated for 1 hour at room temperature. The solvent was removed by compressing the syringe piston. The resin was then allowed to return to its maximum volume by slowly withdrawing the piston. The volume was recorded and the degree of swelling calculated from the following formula:| Degree of swelling (mL g−1) = 10 × (measured volume − 0.12) |
Synthesis of α-amino amide 28
To MeOH or 2-MeTHF (1.5 mL) was added benzhydrylamine (207 μL, 1.2 mmol) and pentanal (128 μL, 1.2 mmol). The resulting solution was agitated at 28 °C for 10 min. To this solution was added benzoic acid (146.5 mg, 1.2 mmol) in anhydrous methanol or 2-MeTHF (3 mL) followed by cyclohexylisocyanide (149 μL, 1.2 mmol). The reaction was agitated for 1.5 h at 28 °C. The mixture was then evaporated in vacuo and the residue dissolved in ethyl acetate (20 mL). The organic solution was washed with 1 M HCl (2 × 10 mL), saturated NaHCO3 (2 × 10 mL) and saturated NaCl (2 × 10 mL), dried (anhydrous MgSO4) and filtered. The organic layer was concentrated in vacuo and the residue purified by flash column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, EtOAc
80, EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE) to give compound 28 (458 mg, 79% in MeOH; 434 mg, 75% in 2-MeTHF) as a yellow oil. Rf: 0.23 (20
PE) to give compound 28 (458 mg, 79% in MeOH; 434 mg, 75% in 2-MeTHF) as a yellow oil. Rf: 0.23 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, EtOAc
80, EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE); νmax(ATR) 3302 w, 2928 s, 2856 w, 1663 s, 1617 m and 1525 cm−1 m; δH 8.05 (1H, br, NH), 7.45–7.18 (15H, m, ArH), 6.27 (1H, s, NCHPh2), 3.81–3.80 (1H, m, NCHC
PE); νmax(ATR) 3302 w, 2928 s, 2856 w, 1663 s, 1617 m and 1525 cm−1 m; δH 8.05 (1H, br, NH), 7.45–7.18 (15H, m, ArH), 6.27 (1H, s, NCHPh2), 3.81–3.80 (1H, m, NCHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3.66–3.57 (1H, m, NHC
O), 3.66–3.57 (1H, m, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.83–1.51 (6H, m, (CH2)3Me), 1.40–0.99 (10H, m, (CH2)5), 0.76 (3H, t J 7.1 Hz, CH3); δC 174.3, 171.0, 137.2, 130.2 129.3, 128.9, 128.7, 128.6, 128.2, 126.3, 77.4, 64.9, 47.6, 32.7, 32.6, 31.4, 29.1, 25.8, 24.6, 24.5, 22.5, 13.9; m/z (ESI+) 505 [(M + Na)+, 100]; Found (ESI+) 505.2820, calculated for C32H38N2NaO2 (M + Na)+ 505.2825.
), 1.83–1.51 (6H, m, (CH2)3Me), 1.40–0.99 (10H, m, (CH2)5), 0.76 (3H, t J 7.1 Hz, CH3); δC 174.3, 171.0, 137.2, 130.2 129.3, 128.9, 128.7, 128.6, 128.2, 126.3, 77.4, 64.9, 47.6, 32.7, 32.6, 31.4, 29.1, 25.8, 24.6, 24.5, 22.5, 13.9; m/z (ESI+) 505 [(M + Na)+, 100]; Found (ESI+) 505.2820, calculated for C32H38N2NaO2 (M + Na)+ 505.2825.
Deprotection of α-amino amide 28
TFA (2 mL) was added to amide 28 (100 mg, 0.2 mmol) and the solution agitated for 4 hours at room temperature. The reaction mixture was evaporated in vacuo and the residue purified by flash column chromatography (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, EtOAc
70, EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE) to give compound 29 as a white solid (44 mg, 67%). Mp 163–163.5 °C; νmax(ATR) 3264 m, 3080 w, 2934 m, 2858 w, 1659 w, 1632 s and 1551 cm−1 m; Rf: 0.25 (30
PE) to give compound 29 as a white solid (44 mg, 67%). Mp 163–163.5 °C; νmax(ATR) 3264 m, 3080 w, 2934 m, 2858 w, 1659 w, 1632 s and 1551 cm−1 m; Rf: 0.25 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, EtOAc
70, EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE); δH 7.84–7.81 (2H, m, ArH), 7.52–7.39 (3H, m, ArH), 7.20 (1H, d J 7.2 Hz, NH), 6.66 (1H, d J 6.7 Hz, NH), 4.65 (1H, dd J 14.7, 7.0 Hz, NCHC
PE); δH 7.84–7.81 (2H, m, ArH), 7.52–7.39 (3H, m, ArH), 7.20 (1H, d J 7.2 Hz, NH), 6.66 (1H, d J 6.7 Hz, NH), 4.65 (1H, dd J 14.7, 7.0 Hz, NCHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3.80–3.71 (1H, m, NCH), 1.93–1.56 (6H, m, (CH2)3Me), 1.40–1.09 (10H, m, (CH2)5), 0.85 (3H, t J 7.0 Hz, CH3); δC 170.8, 167.1, 134.0, 131.6, 128.5, 127.1, 53.6, 48.3, 33.0, 32.8 (2 peaks), 32.7, 27.6, 25.4, 24.7, 22.5, 13.9; m/z (ESI+) 339 [(M + Na)+, 100]; Found (ESI+) 339.2040, calculated for C19H28N2NaO2 (M + Na)+ 339.2043.
O), 3.80–3.71 (1H, m, NCH), 1.93–1.56 (6H, m, (CH2)3Me), 1.40–1.09 (10H, m, (CH2)5), 0.85 (3H, t J 7.0 Hz, CH3); δC 170.8, 167.1, 134.0, 131.6, 128.5, 127.1, 53.6, 48.3, 33.0, 32.8 (2 peaks), 32.7, 27.6, 25.4, 24.7, 22.5, 13.9; m/z (ESI+) 339 [(M + Na)+, 100]; Found (ESI+) 339.2040, calculated for C19H28N2NaO2 (M + Na)+ 339.2043.
Solid-supported synthesis of α-amino amide 29
Resin with an Fmoc-protected Rink linker (100 mg) with capacity given in Table 4 was allowed to swell in DMF (2 mL) for 30 min. Then, a 20% (v/v) solution of piperidine in DMF (2 mL) was added and the resin agitated for 10 min. The resin was filtered and treated a second time with a 20% solution of piperidine in DMF (2 mL) for 10 min. The deprotected resin was then filtered, washed with DMF (2 mL) and CH2Cl2 (2 mL) and dried in vacuo. A sample was subjected to the Kaiser test35 to confirm the presence of primary amines. The remaining resin was suspended in MeOH or 2-MeTHF (2 mL) for 30 min. Pentanal (10 eq. based on the resin loading given in Table 4) was added to the swollen resin and the reaction mixture was agitated for 10 min. Benzoic acid (10 eq. based on the resin loading given in Table 4) and cyclohexylisocyanide (10 eq. based on the resin loading given in Table 4) were added and the resin was agitated for 18 h. The resin was then washed with DMF (5 × 5 mL) and CH2Cl2 (5 × 5 mL) and dried under vacuum. The resin was then treated with a 30% solution of TFA in CH2Cl2 (2 mL) for 3 h at RT. Resin was removed by filtration and the filtrate was concentrated in vacuo. The residue was purified by flash column chromatography (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, EtOAc
70, EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE) to give compound 29 as a white solid in the yields quoted in Table 4. Analytical and spectroscopic data were identical to those reported for compound 29 prepared by a solution-phase synthesis.
PE) to give compound 29 as a white solid in the yields quoted in Table 4. Analytical and spectroscopic data were identical to those reported for compound 29 prepared by a solution-phase synthesis.
Conclusions
When analysed from a green perspective, solid-phase synthesis has the potential to reduce the use of chemicals and energy during reaction work-up and product purification by replacing acid–base washes and chromatography with a simple resin filtration. However, the resin also has to be considered as an auxiliary reagent and whilst it can in principle often be reused in practice this is rarely done. The other green benefit of solid-supported synthesis however is that it facilitates the move from batch to flow reactions. Flow reactions occur under steady state conditions in which energy transfer, reactant composition, product yield and product purity are all time-constant. This can allow the reaction conditions to be optimised to produce purer product that can be obtained in batch reactions and hence reduces waste associated with side product formation and product purification.The solvent is usually the major component (by mass) of any chemical reaction and hence replacement of conventional petrochemical derived solvents by greener, more sustainably sourced ones is a high priority for a chemicals industry keen to improve its environmental impact. In this work we have shown that out of nine resins developed for solid phase synthesis in conventional solvents, eight would swell to an appropriate extent in at least some of 25 green solvents investigated. We have also shown that a computational approach can be used to predict which green solvents (and solvent mixtures) will (and will not) swell a particular resin based on the use of a training set of solvents to determine the dispersion, hydrogen bonding and dipolar energies for the ideal solvent to swell a particular resin and then determining how close the corresponding energies for a particular solvent are to these ideal values. The experimental and modelling work on resin swelling was also validated by an experimental study of solid-phase Ugi reactions carried out with resin/solvent combinations that were predicted to be both suitable and unsuitable.
The resin swelling results reported herein should be equally applicable to other solid-phase syntheses carried out on the resins/solvents studied and the methodology we have developed can be applied to other resins and green solvents. In this context, solid-phase synthesis is sometimes carried out at elevated temperatures often with microwave heating. To assist in green solvent selection, the boiling points of solvents 1–24 are included in Fig. 2. Resins based on crosslinked polystyrene, PEG-grafted polystyrene and crosslinked PEG have all been used at temperatures of 86–200 °C (ref. 36) and polystyrene based resins are known to be stable to at least 250 °C.37
Acknowledgements
The authors thank EPSRC (grant number EP/M506680/1) for a studentship to SL.Notes and references
- R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233–1246 CrossRef CAS.
- D. J. C. Constable, C. Jimenez–Gonzalez and R. K. Henderson, Org. Process Res. Dev., 2007, 11, 133–137 CrossRef CAS.
- A. Benazzouz, L. Moity, C. Pierlot, M. Sergent, V. Molinier and J.-M. Aubry, Ind. Eng. Chem. Res., 2013, 52, 16585–16597 CrossRef CAS.
- Regulation (EC) no. 1907/2006 of the European Parliament and of the Council of 18 December 2006.
- (a) K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC; (b) D. Prat, O. Pardigon, H. W. Flemming, S. Letestu, V. Ducandas, P. Isnard, E. Guntrum, T. Senac, S. Ruisseau, P. Cruciani and P. Hosek, Org. Process Res. Dev., 2013, 17, 1517–1525 CrossRef CAS; (c) C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- A. Amelio, G. Genduso, S. Vreysen, P. Luis and B. Van der Bruggen, Green Chem., 2014, 16, 3045–3063 RSC.
- E. Atherton and R. C. Sheppard, Solid-phase peptide synthesis a practical approach, IRL Press at Oxford University Press, Oxford, 1989 Search PubMed.
- K. J. Barlow, V. Bernabeu, X. Hao, T. C. Hughes, O. E. Hutt, A. Polyzos, K. A. Turner and G. Moad, React. Funct. Polym., 2015, 96, 89–96 CrossRef CAS.
- R. B. Merrifield, J. Am. Chem. Soc., 1963, 85, 2149–2154 CrossRef CAS.
- C. Braunshier and C. Hametner, QSAR Comb. Sci., 2007, 26, 908–918 CAS.
- P. H. Toy and K. D. Janda, Tetrahedron Lett., 1999, 40, 6329–6332 CrossRef CAS.
- S.-S. Wang, J. Am. Chem. Soc., 1973, 95, 1328–1333 CrossRef CAS PubMed.
- W. Li and B. Yan, J. Org. Chem., 1998, 63, 4092–4097 CrossRef CAS.
- H. Rink, Tetrahedron Lett., 1987, 28, 3787–3790 CrossRef CAS.
- O. W. Gooding, S. Baudart, T. L. Deegan, K. Heisler, J. W. Labadie, W. S. Newcomb, J. A. Porco Jr. and P. van Eikeren, J. Comb. Chem., 1999, 1, 113–122 CrossRef CAS.
- M. Bagheri, M. Beyermann and M. Dathe, Antimicrob. Agents Chemother., 2009, 53, 1132–1141 CrossRef CAS PubMed.
- J. H. Adams, R. M. Cook, D. Hudson, V. Jammalamadaka, M. H. Lyttle and M. F. Songster, J. Org. Chem., 1998, 63, 3706–3716 CrossRef CAS.
- F. García-Martín, M. Quintanar-Audelo, Y. García-Ramos, L. J. Cruz, C. Gravel, R. Furic, S. Côté, J. Tulla-Puche and F. Albericio, J. Comb. Chem., 2006, 8, 213–220 CrossRef PubMed.
- http://www.cem.com/spheritide.html . Accessed on 9 June 2016.
- R. Santini, M. C. Griffith and M. Qi, Tetrahedron Lett., 1998, 39, 8951–8954 CrossRef CAS.
- (a) B. Schäffner, F. Schäffner, S. P. Verevkin and A. Börner, Chem. Rev., 2010, 110, 4554–4581 CrossRef PubMed; (b) M. North, F. Pizzato and P. Villuendas, ChemSusChem, 2009, 2, 862–865 CrossRef CAS PubMed; (c) M. North and P. Villuendas, Org. Lett., 2010, 12, 2378–2381 CrossRef CAS PubMed; (d) W. Clegg, R. W. Harrington, M. North, F. Pizzato and P. Villuendas, Tetrahedron: Asymmetry, 2010, 21, 1262–1271 CrossRef CAS; (e) M. Morcillo, M. North and P. Villuendas, Synthesis, 2011, 1918–1925 CAS; (f) C. Beattie, M. North and P. Villuendas, Molecules, 2011, 16, 3420–3432 CrossRef CAS PubMed.
- J. Sherwood, M. De bruyn, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC.
- (a) M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514–1539 RSC; (b) C. Martín, G. Fiorani and A. W. Kleij, ACS Catal., 2015, 5, 1353–1370 CrossRef; (c) A. C. Kathalikkattil, R. Babu, J. Tharun, R. Roshan and D.-W. Park, Catal. Surv. Asia, 2015, 19, 223–235 CrossRef CAS; (d) B. Yu and L.-N. He, ChemSusChem, 2015, 8, 52–62 CrossRef CAS PubMed; (e) M. Cokoja, M. E. Wilhelm, M. H. Anthofer, W. A. Herrmann and F. E. Kühn, ChemSusChem, 2015, 8, 2436–2454 CrossRef CAS PubMed; (f) B.-H. Xu, J.-Q. Wang, J. Sun, Y. Huang, J.-P. Zhang, X.-P. Zhang and S.-J. Zhang, Green Chem., 2015, 17, 108–122 RSC.
- (a) I. S. Metcalfe, M. North, R. Pasquale and A. Thursfield, Energy Environ. Sci., 2010, 3, 212–215 RSC; (b) M. North, B. Wang and C. Young, Energy Environ. Sci., 2011, 4, 4163–4170 RSC; (c) A. Barthel, Y. Saih, M. Gimenez, J. D. A. Pelletier, F. E. Kühn, V. D'Elia and J.-M. Basset, Green Chem., 2016, 18, 3116–3123 RSC.
- G. W. Huber and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369–1379 CrossRef CAS PubMed.
- (a) M. Honda, M. Tamura, Y. Nakagawa, S. Sonehara, K. Suzuki, K.-I. Fujimoto and K. Tomishige, ChemSusChem, 2013, 6, 1341–1344 CrossRef CAS PubMed; (b) I. Prymak, V. N. Kalevaru, S. Wohlrab and A. Martin, Catal. Sci. Technol., 2015, 5, 2322–2331 RSC; (c) J. A. Castro-Osma, J. W. Comerford, S. Heath, O. Jones, M. Morcillo and M. North, RSC Adv., 2015, 5, 3678–3685 RSC.
- M. A. Martín-Luengo, M. Yates, M. J. Martínez Domingo, B. Casal, M. Iglesias, M. Esteban and E. Ruiz-Hitzky, Appl. Catal., B, 2008, 81, 218–224 CrossRef.
- (a) J. Kothandaraman, A. Goeppert, M. Czaun, G. A. Olah and G. K. S. Prakash, J. Am. Chem. Soc., 2016, 138, 778–781 CrossRef CAS PubMed; (b) P. P. Van Uytvanck, B. Hallmark, G. Haire, P. J. Marshall and J. S. Dennis, ACS Sustainable Chem. Eng., 2014, 2, 1098–1105 CrossRef CAS; (c) Y. Soma, K. Inokuma, T. Tanaka, C. Ogino, A. Kondo, M. Okamoto and T. Hanai, J. Biosci. Bioeng., 2012, 114, 80–85 CrossRef CAS PubMed.
- D. G. Blackmond, A. Armstrong, V. Coombe and A. Wells, Angew. Chem., Int. Ed., 2007, 46, 3798–3800 CrossRef CAS PubMed.
- Data in ESI.‡.
- HSPiP software (4th edn 4.1.04, developed by Abbott, Hansen and Yamamoto): http://www.hansen-solubility.com/index.php?id=11.
- (a) A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef CAS; (b) S. Marcaccini and T. Torroba, Nat. Protoc., 2007, 2, 632–639 CrossRef CAS PubMed.
- E. Kaiser, R. L. Colescot, C. D. Bossinge and P. I. Cook, Anal. Biochem., 1970, 34, 595–598 CrossRef CAS PubMed.
- (a) A. Stadler and C. O. Kappe, Eur. J. Org. Chem., 2001, 919–925 CrossRef CAS; (b) B. Bacsa, K. Horváti, S. Bösze, F. Andreae and C. O. Kappe, J. Org. Chem., 2008, 73, 7532–7542 CrossRef CAS PubMed; (c) B. Bacsa, S. Bösze and C. O. Kappe, J. Org. Chem., 2010, 75, 2103–2106 CrossRef CAS PubMed.
- (a) A. Bhatti, J. A. Davies, D. Dollimore and A. Sood, Thermochim. Acta, 1985, 87, 211–218 CrossRef CAS; (b) G. F. Levchik, K. Si, S. V. Levchik, G. Camino and C. A. Wilkie, Polym. Degrad. Stab., 1999, 65, 395–403 CrossRef CAS; (c) Y. Li, Y. Fan and J. Ma, Polym. Degrad. Stab., 2001, 73, 163–167 CrossRef CAS.
Footnotes |
| † Dedicated to James Clark, founder of the Green Chemistry Centre of Excellence and founding editor of Green Chemistry, on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Reproducibility of resin swelling, graphs and tables of solvent modelling outputs and experimental verification of modelling predictions. See DOI: 10.1039/c6gc03147a |
| This journal is © The Royal Society of Chemistry 2017 |