A deeper shade of green: inspiring sustainable drug manufacturing†
Frank
Roschangar
*a,
Juan
Colberg
b,
Peter J.
Dunn‡
c,
Fabrice
Gallou
d,
John D.
Hayler
e,
Stefan G.
Koenig
f,
Michael E.
Kopach
g,
David K.
Leahy
h,
Ingrid
Mergelsberg
i,
John L.
Tucker§
j,
Roger A.
Sheldon
kl and
Chris H.
Senanayake
a
aChemical Development, Boehringer Ingelheim Pharmaceuticals, Ridgefield, Connecticut 06877, USA. E-mail: frank.roschangar@boehringer-ingelheim.com
bPharmaceutical Sciences - Worldwide Research & Development, Pfizer, Groton, Connecticut 06340, USA
cPfizer, Sandwich, UK
dChemical & Analytical Development, Novartis Pharma, 4002 Basel, Switzerland
eAPI Chemistry, GlaxoSmithKline Medicines Research Centre, Stevenage, SG1 2NY, UK
fSmall Molecule Process Chemistry, Genentech, a Member of the Roche Group, South San Francisco, California 94080, USA
gSmall Molecule Design and Development, Eli Lilly and Company, Indianapolis, Indiana 46285, USA
hChemical and Synthetic Development, Bristol-Myers Squibb, New Brunswick, New Jersey 08903, USA
iProcess Chemistry, Merck, Rahway, New Jersey 07065, USA
jProcess Development, Amgen, Thousand Oaks, California 91320, USA
kMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Johannesburg, South Africa
lDelft University of Technology, 2628 BL Delft, Netherlands
First published on 22nd November 2016
Abstract
Green and sustainable drug manufacturing go hand in hand with forward-looking visions seeking to balance the long-term sustainability of business, society, and the environment. However, a lack of harmonization among available metrics has inhibited opportunities for green chemistry in industry. Moreover, inconsistent starting points for analysis and neglected complexities for diverse manufacturing processes have made developing objective goals a challenge. Herein we put forward a practical strategy to overcome these barriers using data from in-depth analysis of 46 drug manufacturing processes from nine large pharmaceutical firms, and propose the Green Aspiration Level as metric of choice to enable the critically needed consistency in smart green manufacturing goals. In addition, we quantify the importance of green chemistry in the often overlooked, yet enormously impactful, outsourced portion of the supply chain, and introduce the Green Scorecard as a value added sustainability communication tool.
Introduction
Our mission is to enable broad adoption of green chemistry1–4 within the pharmaceutical industry for the benefit of society, industry, and the environment.5,6 In order to create a triple win among these stakeholders, the dynamic interactions and resource flows between them must be carefully balanced to achieve a sustainable civilization (ESI Fig. 1†).7 Social responsibility, including environmental protection, must be seen as a source of opportunity for reputation building, innovation and competitive advantage rather than a cost constraint.8–10 Green chemistry is precisely the means to delivering these benefits11,12 while also stimulating a culture of scientific discovery.13,14 However, broad adoption of green chemistry within industry has been hampered by the lack of objective goals and harmonized metrics. Breaking down these barriers and achieving our vision necessitates a two-level strategy. On one level, we need to unify the many metric-based approaches and transform them into tangible and uncomplicated goals. Additionally, we need to create a good communication strategy for alignment across the pharmaceutical industry to embrace and foster such agreed-upon goals.First, to tackle metrics unification, we take advantage of the recently introduced Green Aspiration Level (GAL).15 The GAL is not simply another process metric, but rather represents the first substantial green efficiency goal for any manufacturing process to a given drug. It has the distinct advantage over other traditional waste metrics of defining the expected environmental impact for producing any pharmaceutical agent by considering the complexity of its ideality-adjusted synthesis route.16 With this quantifiable descriptor, process greenness becomes measurable, and as such can deliver manageable and credible green chemistry process goals. This is in line with the proven management adage “you can only manage what you can measure”. We note that the GAL assesses process waste and as such intentionally discounts process energy, safety, and environmental impact, which are integral parts of the complementary Life Cycle Analysis (LCA).17–22 In fact, GAL consensus analysis starting points will streamline any LCA approach, and we therefore envision integration of the two concepts in the future.
Second, to realize the complex goal of aligning industry and broadly implementing the GAL methodology, we explored the concept within two leading green chemistry industry consortia. The International Consortium for Innovation & Quality in Pharmaceutical Development (IQ, https://iqconsortium.org/initiatives/working-groups/green-chemistry) and the ACS Green Chemistry Institute Pharmaceutical Roundtable (ACS GCI PR, https://www.acs.org/content/acs/en/greenchemistry/industry-business/pharmaceutical.html) strongly support the Green Aspiration Level as a valuable tool to evaluate best green chemistry choices. Consequently, we decided to showcase the GAL to assess green drug manufacture. For this purpose, we streamlined the methodology for consistency (ESI Discussion 1†) and defined a single smart process complexity-based waste goal (ESI Discussion 2†).
Using the green aspiration level to rate relative process greenness in drug manufacture
An overview of the GAL methodology is given in ESI Discussion 1 and 2.† A key technical advantage is its ease of use. It takes just four intuitive and quick steps to complete a process analysis and determine and rate Relative Process Greenness (RPG) (Chart 1). Most of the effort goes into the first step of determining process waste via complete E factor (cEF)15,23 or Process Mass Intensity (PMI),24 while the last three steps can be completed by simple calculations. The RPG can be effectively visualized via our new Green Scorecard vide infra.We carried out an in-depth analysis of 46 manufacturing processes from nine large pharmaceutical firms with good distribution across the drug lifecycle in early development, late development, and commercial manufacturing. The results of our analysis are summarized in Table 1 and detailed in ESI Table 1.† Our process waste data correlate well with those from 2008 data by the ACS GCI PR25 (ESI Table 2†) that had been used to establish the original process step waste goal, termed transformation GAL or tGAL. Further coherence with the 2008 data is observed with respect to solvent and water utilization. The ACS GCI PR reported 86% average water and solvent utilization, while our dataset reveals 87–91% (ESI Fig. 2†). Comparison of simple E factor (sEF, ESI eqn (1)†) and cEF values are a helpful indicator for relative solvent use and thus waste reduction potential.
| Phase of drug manufacturing | N | Complexity | sEF [kg kg−1] | cEF [kg kg−1] | GAL [kg kg−1] | RPG | % cEF litd | % (organic solvents + water) |
|---|---|---|---|---|---|---|---|---|
| a RPG calculated based on updated tGAL = 26 kg kg−1. b All figures represent the means. c N = number of manufacturing process data sets. d % waste derived from literature procedures to fulfill $100 per mol process analysis starting point requirement. e Campaigns making drug supplies for up to Phase IIa/Proof of Concept clinical trials. f Campaigns supporting Phase IIb clinical trials up to registration. g Campaigns providing market supplies. | ||||||||
| Early developmente | 16 | 9.4 | 90 | 793 | 245 | 49% | 21% | 87% |
| Late developmentf | 15 | 8.0 | 34 | 308 | 208 | 96% | 17% | 91% |
| Commercialg | 12 | 5.9 | 15 | 156 | 154 | 132% | 20% | 89% |
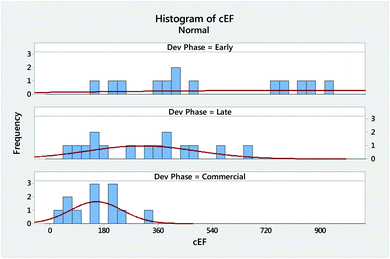
Diving deeper into our analysis, a dissection of the cEF waste data shows both the expected reduction in process waste and narrowing distribution of cEF with advancing project phase (Fig. 1). The cEF values of three early development projects included in the analysis are outside the display region with values of 1361, 1744, and 2746.
With implementation of the US $100 mol−1 process analysis starting points (ESI Discussion 1†), and the resulting need to consult literature procedures for raw materials that do not meet this requirement, the 2016 data are expected to show more waste per kg of produced drug, and this is principally observed. In fact, without the standardized starting point rule, an average of 20% process waste would have been excluded from our results, and overall process waste understated accordingly. Thus, we deemed it imperative to apply our new commercial process data to update the tGAL from 19 to 26 kg kg−1 and utilize the revised value for Relative Process Greenness (RPG) evaluation (ESI Discussion 2†).
As one would expect, the average RPG (ESI eqn (2)†) significantly improves and increases over the course of development into commercialization from 49 to 96 and then to 132%. The RPG for the average commercial process is 100%. If the GAL goal had been in place prior to our analysis, it would have been realized (RPG ≥ 100%) with 6 of the 12 analyzed commercial processes (ESI Table 1,† entries 32–37). Our data therefore establish the GAL as the first meaningful and achievable SMART (Specific, Measurable, Achievable, Results-based, Time-bound) green chemistry process goal.
Our study also helps refine the scope of the tGAL goal. For example, three late phase and commercial drugs with highly solvent volume-intensive manufacturing processes typically associated with New Biological Entities (NBEs), polypeptides, carbohydrates, and drug conjugates, would have abysmal RPG ranging from 4 to 20% (ESI Table 1,† entries 44–46) when using the new tGAL value. This emphasizes the need for distinct tGAL for these classes of compounds. Of course, the GAL methodology still applies per se, but a new tGAL value must be established in order to enable smart green chemistry process goals of these compound classes. Thus, the tGAL discussed herein is pertinent only to small molecule New Chemical Entity (NCE) drugs.
The fact that we have simplified GAL methodology with a single commercial tGAL goal brings about relatively low RPG values for early and late development drug processes. Since it is motivationally important to maintain achievable goals across all phases and enable managers and teams to set and strive for smart green chemistry goals, communicate green chemistry process achievements, and encourage green chemistry throughout the entire drug development lifecycle, we create a fair phase-dependent ranking system that is described with our communication strategy vide infra.
Importance of green chemistry in the supply chain
The external manufacturing supply chain is receiving increased attention in conjunction with green chemistry considerations.26,27 Since “[the supply chain] is where the greatest impact [of manufacturing waste] could be”,28 and the cost of drug manufacturing correlates to the amount of generated waste and environmental impact, effective management of the supply chain is expected to deliver efficiencies and economic value. As a consequence, in recent years, stakeholders in the pharmaceutical sector ranging from investors and shareholders to customers have increased pressure on pharmaceutical firms to better track and optimize sustainable supply chain manufacturing performance.But how important is it exactly to manage green chemistry in the supply chain? What is the impact of the pharmaceutical manufacturing supply chain on overall drug process waste generation? How green is the supply chain? To our knowledge we are the first to quantitatively address these important questions.
We include literature step waste in external process waste analysis (ESI Fig. 3†). Our results show that 41–61% or about half of the drug manufacturing process waste is generated externally (Table 2). Thus, green chemistry in the supply chain is vitally important. From the data set we also derive that Relative Process Greenness (RPG) of the overall process (internal and external manufacture) is similar to the external, indicating comparable green process performance, and opportunities to improve, within the pharmaceutical firms and their supply chains.
| Phase of drug manufacturing | % External waste | External RPG | Overall process RPG |
|---|---|---|---|
| a All figures represent the means. | |||
| Early development | 41% | 58% | 49% |
| Late development | 61% | 109% | 96% |
| Commercial | 44% | 145% | 132% |
However, until now managing external green chemistry has proven difficult due to the lack of simple and fair benchmarking concepts.29 Undoubtedly our data show that the new GAL methodology is ideally suited to also establish smart external green process manufacturing goals. For example, the GAL and RPG can readily be integrated into procurement systems and category scorecards, possibly via the green scorecard introduced vide infra. This will allow pharmaceutical buyers to monitor and reward supplier performance and improvements against smart green chemistry goals, and catalyze external green chemistry efforts with constructive feedback based on quantitative and standardized RPG data. In addition, suppliers are empowered to quantify their contributions and evaluate their own supply chain. Overall, the GAL methodology has the potential to inspire environmental impact reductions within the supply chain, stimulate external green innovation, and foster best green chemistry practices from “cradle to grave”.
Communicating green chemistry value added: the green scorecard
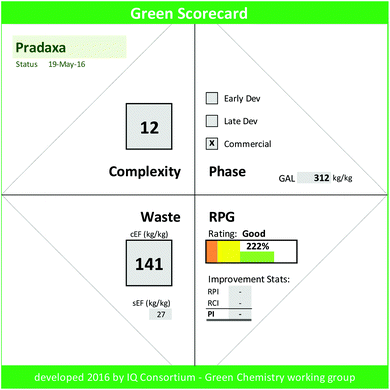
Hitherto we have outlined the importance of smart green chemistry manufacturing goals and harmonized metrics as they relate to broad chemistry adoption. To fully convey the opportunities and improvements, however, we require an effective strategy to integrate GAL goals within our firms and across industry. We believe that it is paramount to communicate the value added impact of green chemistry improvements in a simple and effective way. Thus, waste reduction in conjunction with RPG and process improvements need to be appropriately highlighted. Bearing these goals in mind, the IQ Green Chemistry working group developed the Green Scorecard. The scorecard captures the key facts of the current manufacturing process, the lifecycle status of the drug, process complexity, and the GAL goal, and presents green overall process improvement statistics (PI, ESI eqn (3)†) that were achieved through both process waste reductions (RPI, ESI eqn (4)†) and innovative complexity improvements (RCI, ESI eqn (5)†). It effectively visualizes RPG via “traffic light gauge” and shows the rating of the manufacturing process relative to the project status. A Green Scorecard example (Fig. 2) is shown for the commercial Pradaxa drug manufacture (ESI Fig. 4†).30The calculator template has been made available via free download from the IQ GC website (https://iqconsortium.org/initiatives/projects/green-aspiration-level). To use the Green Scorecard calculator, one simply inputs project name and phase, complexity, sEF, and cEF for one or more completed campaign in order to obtain the calculated RPG and its rating. The impact of innovation and waste reductions via RPI, RCI, and PI are auto-calculated if two or more campaigns are entered (ESI Discussion 3†).
The phase-dependent Green Scorecard ratings are derived from our dataset via probability plots of RPG at the 90, 70, and 40 percentile levels (ESI Fig. 5†). We assign four process greenness ratings of excellent = 90 percentile, good = 70 percentile, average = 40 percentile, and below average for the bottom 40% RPG values. This results in the phase-dependent RPG ratings matrix of Table 3.
Thus, the Pradaxa process with a RPG of 222% falls into the commercial 70 percentile, i.e. is estimated greener than 70% of commercial industrial process in terms of waste quantity and rated “good”.
The Green Scorecard will be the integral part of our green chemistry implementation and communication strategy. It holds the potential to cascade broad adoption of the GAL throughout the pharmaceutical industry and its supply chain into process chemists and engineers daily routine, since it forcefully yet fairly communicates the objective value added of green process chemistry via quantitative and goal-driven data, unlike any other green chemistry metric to date.
Conclusion
In summary, we have demonstrated that process greenness can be objectively measured and rated via RPG, making green drug manufacturing fully manageable. With our vision to unfold the full potential of green chemistry, we have put forward a unified strategy to (1) harmonize green chemistry manufacturing metrics through consistent starting points, and (2) establish universal and tangible green manufacturing goals that consider process complexity via the Green Aspiration Level. However, “the best strategic plans in the world are not likely to be successful if they are not effectively communicated to those who must implement them” (quote from Jake Laban and Jack Green). Consequently, we developed the Green Scorecard to inform and initiate the cascade of our strategy throughout the industry including the significant supply chain.It is conceivable that with increasing acceptance the GAL will become a mainstream tool beyond pharmaceutical manufacturing and find application as key criterion in green chemistry award selection processes. We consider the GAL methodology complementary to the LCA tool from the ACS GCI PR mentioned vide supra, and can envision integration of both concepts. In fact, incorporation of GAL's consensus analysis starting points will enhance consistency of the streamlined LCA approach. “History teaches us that most breakthrough innovations are … [integrated] … through networks”,31 so we will continue to work through the IQ and ACS GCI PR consortia to foster our vision.
Acknowledgements
F. R. thanks W. Samstag for input with Pradaxa's process greenness analysis, and D. R. Fandrick, K. R. Fandrick, J. C. Lorenz, M. A. Marsini, J. T. Reeves, R. Soyka, H. Wu, and Y. Zhang for E factor calculations and project data. F. G. thanks U. Onken, W. Shieh, H. Scheidat, and A. Koettgen. P. J. D. thanks C. P. Ashcroft, G. Assaf and L. J. Harris for project data and environmental metrics analysis. J. H. thanks L. Boulton, J. Lim, L. Liu, and V. Ironmonger for their assistance with PMI calculations.Notes and references
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed.
- M. Poliakoff and P. Licence, Sustainable Technology: Green Chemistry, Nature, 2007, 450, 810–812 Search PubMed.
- C.-J. Li and B. M. Trost, Green Chemistry for Chemical Synthesis, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197–13202 Search PubMed.
- P. T. Anastas, B. Han, W. Leitner and M. Poliakoff, “Happy Silver Anniversary”: Green Chemistry at 25, Green Chem., 2016, 18, 12–13 Search PubMed.
- M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Green chemistry: Science and politics of change, Science, 2002, 297, 807–810 Search PubMed.
- R. Noyori, Synthesizing our Future, Nat. Chem., 2009, 1, 5–6 Search PubMed.
- J. Fiksel, T. Eason and H. Frederickson, A Framework for Sustainability Indicators at EPA, EPA/600/R/12/687, Environmental Protection Agency, Washington, DC, 2013.
- M. E. Porter and M. R. Kramer, Strategy and Society: The Link between, Competitive Advantage and Corporate Social Responsibility, Harv. Bus. Rev., 2006, 84, 78–92 Search PubMed.
- S. A. Matlin, G. Mehta, H. Hopf and A. Krief, One-World Chemistry and Systems Thinking, Nat. Chem., 2016, 8, 393–398 Search PubMed.
- M. Kessel, Restoring the Pharmaceutical Industry's Reputation, Nat. Biotechnol., 2014, 32, 983–990 Search PubMed.
- D. K. Leahy, et al., Seven Important Elements for an Effective Green Chemistry Program: An IQ Consortium Perspective, Org. Process Res. Dev., 2013, 17, 1099–1109 Search PubMed.
- J. L. Tucker and M. M. Faul, Drug Companies Must Adopt Green Chemistry, Nat. News, 2016, 534, 27–28 Search PubMed.
- P. T. Anastas and J. B. Zimmermann, Innovations in Green Chemistry and Green Engineering, Springer, New York, 2013 Search PubMed.
- J. H. Clark, T. J. Farmer, L. Herrero-Davila and J. Sherwood, Circular Economy Design Considerations for Research and Process Development in the Chemical Sciences, Green Chem., 2016, 18, 3914–3934 Search PubMed.
- F. Roschangar, R. A. Sheldon and C. H. Senanayake, Overcoming Barriers to Green Chemistry in the Pharmaceutical Industry - the Green Aspiration Level Concept, Green Chem., 2015, 17, 752–768 Search PubMed.
- T. Gaich and P. S. Baran, Aiming for the Ideal Synthesis, J. Org. Chem., 2010, 75, 4657–4673 Search PubMed.
- A. D. Curzons, C. Jimenez-Gonzalez, A. Duncan, D. J. C. Constable and V. L. Cunningham, Fast Life-Cycle Assessment of Synthetic Chemistry (FLASC™) Tool, Int. J. Life Cycle Assess., 2007, 12, 272–280 Search PubMed.
- L. M. Tufvesson, P. Tufvesson, J. Woodley and P. Börjesson, Life Cycle Assessment in Green Chemistry: Overview of Key Parameters and Methodological Concerns, Int. J. Life Cycle Assess., 2013, 18, 431–444 Search PubMed.
- C. Jiménez-González, et al., Expanding the Boundaries: Developing a Streamlined Tool for Eco-Footprinting of Pharmaceuticals, Org. Process Res. Dev., 2013, 17, 239–246 CrossRef.
- C. Jiménez-González and M. R. Overcash, The Evolution of Life Cycle Assessment in Pharmaceutical and Chemical Applications – a Perspective, Green Chem., 2014, 16, 3392–3400 Search PubMed.
- D. Cespi, et al., Life Cycle Inventory Improvement in the Pharmaceutical Sector: Assessment of the Sustainability Combining PMI and LCA tools, Green Chem., 2015, 17, 3390–3400 Search PubMed.
- C. R. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Towards a Holistic Approach to Metrics for the 21st Century Pharmaceutical Industry, Green Chem., 2015, 17, 3111–3121 Search PubMed.
- R. A. Sheldon, The E Factor: Fifteen Years On, Green Chem., 2007, 9, 1273–1283 Search PubMed.
- C. Jiménez-González, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes, Org. Process Res. Dev., 2011, 15, 912–917 Search PubMed.
- ACS GCI Pharmaceutical Roundtable, Convergent PMI Calculator, http://www.acs.org/content/dam/acsorg/greenchemistry/industriainnovation/roundtable/Convergent-PMI-Presentation.pptx, 2014.
- D. J. C. Constable, Differentiating Yourself from the Competition in the Green Chemistry Supply Chain, http://news.thomasnet.com/IMT/2013/04/04/differentiating-yourself-from-the-competition-in-the-green-chemistry-supply-chain, 2013.
- T. Fenenelly, Associates, Advancing Green Chemistry: Barriers to Adoption and Ways to Accelerate Green Chemistry in Supply Chains, http://www.greenchemistryandcommerce.org/documents/Advancing-Green-Chemistry-Report-June2015.pdf, 2015.
- A. Ward, Green chemistry: Pharmaceutical Companies Try to Clean up Their Act, http://www.ft.com/intl/cms/s/0/20265526-e58c-11e3-a7f5-00144feabdc0.html#axzz3vAhxCYls, 2014.
- W. De Soete, Towards a Multidisciplinary Approach on Creating Value: Sustainability through the Supply Chain and ERP Systems, Systems, 2016, 4, 16–27 Search PubMed.
- C. Filser, et al., Method for Producing an Intermediate Product of Dabigatran Etexilate, WO Patent, 2009153215, 2009; filed 12 Jun. 2009, and issued 23 Dec. 2009 Search PubMed.
- R. Cross, A. Hargadon, S. Parise and R. Thomas, Critical Connections: Driving Rapid Innovation with a Network Perspective, Sloan Management Review and Wall Street Journal Weekend Edition, http://www.robcross.org/pdf/roundtable/networks_and_innovation.pdf, 2008.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6gc02901a |
| ‡ Retired. |
| § Current address: Neurocrine Biosciences, San Diego, California 92130, USA. |
| This journal is © The Royal Society of Chemistry 2017 |