Making H2 from light and biomass-derived alcohols: the outstanding activity of newly designed hierarchical MWCNT/Pd@TiO2 hybrid catalysts†
Abstract
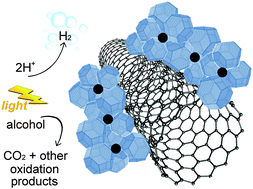
Hydrogen evolution is among the most investigated catalytic processes given the importance of H2 from an industrial and an energy perspective. Achieving H2 production through green routes, such as water splitting or more realistically photoreforming of alcohols, is particularly desirable. In this work, we achieve a remarkable H2 productivity through photoreforming of either ethanol or glycerol as a sacrificial electron donor by employing a hybrid nanocatalyst where the properties of multi-walled carbon nanotubes (MWCNTs), Pd nanoparticles and crystalline TiO2 are optimally merged through appropriate engineering of the three components and an optimised synthetic protocol. Catalysts were very active both under UV (highest activity 25 mmol g−1 h−1) and simulated solar light (1.5 mmol h−1 g−1), as well as very stable. Critical to such high performance is the intimate contact of the three phases, each fulfilling a specific task synergistically with the other components.
- This article is part of the themed collection: Harvesting Renewable Energy with Chemistry


 Please wait while we load your content...
Please wait while we load your content...