Mitigating the effects of high fat diet on the brain and behavior with berry supplementation
Abstract
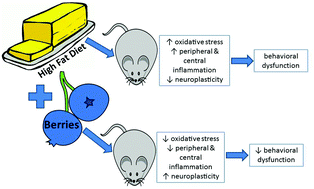
Research on the potential of berries to modulate the effects of high fat diets on the brain and behavior is a relatively small and growing field. This review provides an overview of current findings from animal studies assessing the impact of high fat diets supplemented with blueberries, blackberries, grapes and jaboticaba berries on cognitive performance and neuroprotection. High fat diets are demonstrated to increase brain markers of oxidative stress and inflammation and result in other neural alterations that can contribute to impairments in learning and memory. Berries are rich in bioactive polyphenols and show promise for mitigating the effects of high fat diet. Challenges to systematic research include variability in diet composition and regimens, limitations of predominantly male animal models, and other factors. Links between peripheral inflammation and CNS dysfunction have implications for the understanding of underlying mechanisms and directions for future research.
- This article is part of the themed collection: Berry Health Benefits Symposium


 Please wait while we load your content...
Please wait while we load your content...