The effect of dietary factors on strawberry anthocyanins oral bioavailability
Abstract
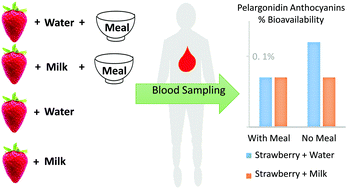
Strawberries are a dietary source of anthocyanins, particularly pelargonidin glycosides. Dietary anthocyanins have received increasing attention among researchers and consumers due to their health benefits. The oral bioavailability of anthocyanins is reported to be low and various dietary factors may influence their oral bioavailability further. Milk is suggested to reduce (poly)phenols’ oral bioavailability. However, the effect of milk on anthocyanin oral bioavailability remains uncertain. Likewise, mixed nutrient meals may influence the oral bioavailability of anthocyanins. Therefore, the purpose of this study was to assess the effect of milk on the oral bioavailability and other pharmacokinetic (PK) variables of strawberry anthocyanins consumed with and without a meal. Nine healthy participants consumed a strawberry beverage prepared in milk or water with a standard meal on two occasions. On two additional occasions, the beverages were given to a subset (n = 4) of participants to determine the impact of the meal on anthocyanin PK variables, including oral bioavailability. Independent of the meal, beverages prepared in milk significantly reduced the peak plasma concentrations (Cmax) of pelargonidin-3-O-glucoside (P-3-G), pelargonidin-glucuronide (PG) and pelargonidin-3-O-rutinoside (P-3-R), as well as the PG and P-3-R area under the curve (AUC) (p < 0.05) compared to beverages prepared in water. Milk did not influence the oral relative bioavailability of pelargonidin anthocyanins under meal conditions; however, the oral relative bioavailability of pelargonidin anthocyanins was reduced by ∼50% by milk under without meal conditions (p < 0.05). Consuming strawberry beverages made with milk and consuming those made with water with and without a meal influenced different aspects of strawberry anthocyanin PKs. The significance of this effect on clinical efficacy requires additional research.
- This article is part of the themed collection: Berry Health Benefits Symposium


 Please wait while we load your content...
Please wait while we load your content...