Exosomal delivery of berry anthocyanidins for the management of ovarian cancer
Abstract
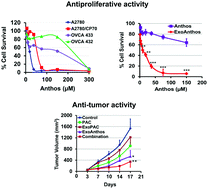
Despite optimal diagnosis and early therapeutic interventions, the prognosis for ovarian cancer patients remains dismal because the efficacy of chemotherapy is limited by the development of resistance and off-site toxicity. Berry bioactives indicate preventive and therapeutic activities against various cancer types. Here, we examined the antiproliferative activity of berry anthocyanidins (Anthos) against drug-sensitive (A2780) and drug-resistant (A2780/CP70, OVCA432 and OVCA433) ovarian cancer cells. These drug-resistant ovarian cancer cell lines overexpress p-glycoproteins (PgP) and show >100-fold resistance to the chemotherapeutic drug cisplatin compared to A2780. We observed a dose-dependent growth inhibition of ovarian cancer cells with the Anthos. Furthermore, the treatment of drug-resistant ovarian cancer (OVCA433) cells with cisplatin in combination with the Anthos (75 μM) resulted in significantly higher cell killing. The cisplatin dose required to achieve this effect was 10 to 15-fold lower than the IC50 of cisplatin alone. However, many plant bioactives including Anthos face the challenge of poor oral bioavailability and stability. Recently, we have developed strategies to overcome these limitations by delivering Anthos via milk-derived exosomes. The exosomal Anthos (ExoAnthos) significantly enhanced the antiproliferative activity against the growth of ovarian cancer cells and inhibited tumor growth more efficiently compared to Anthos alone and a vehicle control. Often patients with cisplatin-resistant tumors retain sensitivity to paclitaxel (PAC). We prepared exosomal formulations of PAC (ExoPAC) for oral delivery as the systemic administration of PAC has severe side effects. ExoPAC delivered orally showed the same therapeutic efficacy as the free PAC delivered intraperitoneally. Finally, we report that the combination of the Anthos and PAC decreased the PgP level in a dose-dependent manner in OVCA432 cells. A significantly enhanced antitumor activity was observed with the combination of ExoPAC and ExoAnthos against A2780 tumor xenografts. Together, our data indicate that the berry Anthos are highly effective against ovarian cancer and that the milk exosomes serve as an excellent nano-carrier to enhance the drug's oral bioavailability for the management of ovarian cancer.
- This article is part of the themed collection: Berry Health Benefits Symposium


 Please wait while we load your content...
Please wait while we load your content...