A serving of blueberry (V. corymbosum) acutely improves peripheral arterial dysfunction in young smokers and non-smokers: two randomized, controlled, crossover pilot studies
Abstract
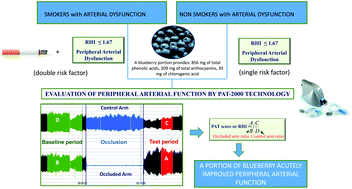
Several studies have documented the important role of polyphenol-rich foods in the modulation of vascular remodelling and function. This study aimed to evaluate the capacity of a single portion of blueberry (V. corymbosum) to acutely improve peripheral arterial dysfunction in a group of young volunteers. Twenty-four healthy males (12 non-smokers and 12 smokers) were recruited for two different randomized, controlled, crossover pilot acute studies. In the first study, non-smokers were exposed to a control treatment (C; 300 mL of water with sugar) and a blueberry treatment (BB; 300 g of blueberry). In the second study, smokers underwent 3 different protocols: (1) – smoking treatment (S); (2) – control treatment (CS; 300 mL of water with sugar + smoking); (3) – blueberry treatment (BS; 300 g of blueberry + smoking). Each treatment (1 day long) was separated by a one week washout period. Blood pressure, peripheral arterial function (reactive hyperemia index, RHI, a marker of endothelial function) and arterial stiffness (digital augmentation index, dAix and dAix normalized by considering a heart rate of 75 bpm, dAix@75) were measured before and after each treatment. In the first study, the consumption of blueberry and control treatment acutely increased peripheral arterial function in the group of non-smokers. The improvement in RHI was higher and significantly different after blueberry treatment compared to the control treatment (54.8 ± 8.4% BB vs. 28.2 ± 8.3% C; p = 0.01). No effects were observed for markers of arterial stiffness, blood pressure and heart rate. Acute cigarette smoke significantly increased blood pressure and heart rate, while no significant effect was registered in peripheral arterial function and stiffness. The intake of blueberry and control treatment before a cigarette did not counteract the increase in blood pressure and heart rate, while it significantly improved peripheral arterial function. In particular, a significant increase was observed following BS (35.2 ± 7.5% RHI; p = 0.02) and CS treatments (34.6 ± 11.9% RHI; p = 0.02) when compared to only smoking treatment. No difference between BS and CS was detected. In conclusion, the intake of blueberry and control treatments acutely improved peripheral arterial dysfunction both in smoker and in non-smoker subjects. Further studies should be performed to confirm the results obtained and reveal the potential mechanisms of blueberry in the improvement of endothelial function.
- This article is part of the themed collection: Berry Health Benefits Symposium


 Please wait while we load your content...
Please wait while we load your content...