The composition of potentially bioactive triterpenoid glycosides in red raspberry is influenced by tissue, extraction procedure and genotype†
Abstract
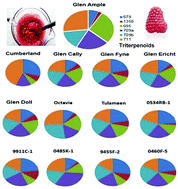
The beneficial effects of consumption of berry fruits on a range of chronic diseases has been attributed (at least in part) to the presence of unique phytochemicals. Recently, we identified novel ursolic acid-based triterpenoid glycosides (TTPNs) in raspberry fruit and demonstrated their survival in human ileal fluids after feeding which confirmed their colon-availability in vivo. In this paper, in vitro digestion studies demonstrated that certain TTPNs were stable under gastrointestinal conditions and confirmed that these components may have been responsible for bioactivity noted in previous studies. Sequential extractions of raspberry puree, isolated seeds and unseeded puree showed that certain TTPN components (e.g. peak T1 m/z 679, and T2 m/z 1358) had different extractabilities in water/solvent mixes and were differentially associated with the seeds. Purified seed TTPNs (mainly T1 and T2) were shown to be anti-genotoxic in HT29 and CCD841 cell based in vitro colonocyte models. Further work confirmed that the seeds contained a wider range of TTPN-like components which were also differentially extractable in water/solvent mixes. This differential extractability could influence the TTPN composition and potential bioactivity of the extracts. There was considerable variation in total content of TTPNs (∼3-fold) and TTPN composition across 13 Rubus genotypes. Thus, TTPNs are likely to be present in raspberry juices and common extracts used for bioactivity studies and substantial variation exists in both content and composition due to genetics, tissue source or extraction conditions, which may all affect observed bioactivity.
- This article is part of the themed collection: Berry Health Benefits Symposium


 Please wait while we load your content...
Please wait while we load your content...