Blueberry supplementation attenuates oxidative stress within monocytes and modulates immune cell levels in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled trial
Abstract
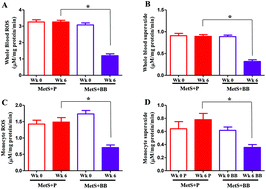
Background: Blueberries (BB) have been shown to improve insulin sensitivity and endothelial function in obese and pre-diabetic humans, and decrease oxidative stress and inflammation, and ameliorate cardio-renal damage in rodents. This indicates that blueberries have a systemic effect and are not limited to a particular organ system. In order for blueberries to exert beneficial effects on the whole body, the mechanism would logically have to operate through modulation of cellular humoral factors. Objective: This study investigated the role of blueberries in modulating immune cell levels and attenuating circulatory and monocyte inflammation and oxidative stress in metabolic syndrome (MetS) subjects. Design: A double-blind, randomized and placebo-controlled study was conducted in adults with MetS, in which they received a blueberry (22.5 g freeze-dried) or placebo smoothie twice daily for six weeks. Free radical production in the whole blood and monocytes, dendritic cell (DC) levels, expression of cytokines in monocytes and serum inflammatory markers were assessed pre- and post-intervention. Results: Baseline free radical levels in MetS subjects’ samples were not different between groups. Treatment with blueberries markedly decreased superoxide and total reactive oxygen species (ROS) in whole blood and monocytes compared to the placebo (p ≤ 0.05). The baseline DC numbers in MetS subjects’ samples in both groups were not different, however treatment with blueberries significantly increased myeloid DC (p ≤ 0.05) and had no effect on plasmacytoid cells. Blueberry treatment decreased monocyte gene expression of TNFα, IL-6, TLR4 and reduced serum GMCSF in MetS subjects when compared to the placebo treatment (p ≤ 0.05). Conclusions: The findings of the current study demonstrate that blueberries exert immunomodulatory effects and attenuate oxidative stress and inflammation in adults with MetS.
- This article is part of the themed collection: Berry Health Benefits Symposium


 Please wait while we load your content...
Please wait while we load your content...