Presence and formation of fluorescence carbon dots in a grilled hamburger†
Abstract
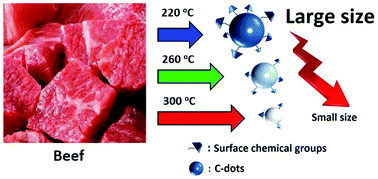
The presence of nanomaterials during food processing has attracted significant concern due to the physicochemical properties of nanomaterials. In this study, the presence and formation of nitrogen-containing fluorescence carbon dots (C-dots) in a grilled hamburger at different temperatures (220, 260, and 300 °C) were investigated during the pyrolysis process. The size and morphology of the C-dots were found to be highly dependent on the heating temperatures, which again affected the functional groups on their surface. The C-dots are strongly fluorescent with multicolor emission accompanied by a gradual decrease in fluorescence intensity. The fluorescence quantum yield of the C-dots produced at 260 °C was measured to be 23.25%. The potential cytotoxicity and biodistribution of the C-dots within live organisms were examined with the mouse osteoblasts cell line and mung bean sprout, respectively. The cell viability after 24 h incubation remained 79% for the C-dots obtained at 300 °C at a concentration of 3.2 mg mL−1, and no obvious phytotoxicity in the growing mung bean sprout was observed. The results showed an increased cytotoxicity of the C-dots formed at higher temperatures.


 Please wait while we load your content...
Please wait while we load your content...