Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids
Abstract
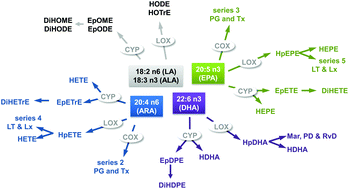
A growing body of evidence suggests that the intake of the long chain omega-3 polyunsaturated fatty acids (n3-PUFA) eicosapentaenoic acid (C20:5 n3, EPA) and docosahexaenoic acid (C22:6 n3, DHA) is linked to beneficial health effects, particularly in the prevention of cardiovascular and inflammatory diseases. Although the molecular mode of action of n3-PUFA is still not fully understood, it is not controversial that a significant portion of the (patho)-physiological effects of PUFA are mediated by their oxidative metabolites, i.e. eicosanoids and other oxylipins. Quantitative targeted oxylipin methods allow the comprehensive monitoring of n3-PUFA supplementation induced changes in the pattern of oxylipins in order to understand their biology. In this short review, results from intervention studies are summarized analyzing >30 oxylipins from different PUFAs in response to n3-PUFA supplementation. The results are not only qualitatively compared with respect to the study design, n3-PUFA dose and trends in the lipid mediators, but also quantitatively based on the relative change in the oxylipin level induced by n3-PUFA. The evaluation of the data from the studies shows that the change in oxylipins generally corresponded to the observed changes in their precursor PUFA, i.e. the lower the individual n3-status at the baseline, the higher the increase in EPA and DHA derived oxylipins. The strongest relative increases were found for EPA derived oxylipins, while changes in arachidonic acid (C20:4 n6, ARA) derived eicosanoids were heterogeneous. After 3–12 weeks of supplementation, similar relative changes were observed in free and total (free + esterified) oxylipins in plasma and serum. Regarding EPA derived oxylipins, the results indicate a trend for a linear increase with dose. However, the interpretation of the quantitative oxylipin patterns between studies is hampered by strong inter-individual variances in oxylipin levels between and also within the studies. In the future, the reason for these varying oxylipin plasma concentrations needs to be clarified in order to understand oxylipin and n3-PUFA biology.


 Please wait while we load your content...
Please wait while we load your content...