Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Long-term assessment of six-stacked scaled-up MFCs treating swine manure with different electrode materials†
A.
Vilajeliu-Pons
 a,
S.
Puig
a,
S.
Puig
 *a,
I.
Salcedo-Dávila
b,
M. D.
Balaguer
*a,
I.
Salcedo-Dávila
b,
M. D.
Balaguer
 a and
J.
Colprim
a and
J.
Colprim
 a
a
aLaboratory of Chemical and Environmental Engineering (LEQUiA), Institute of the Environment, C/Maria Aurèlia Capmany, 69, Facultat de Ciències, University of Girona, E-17003 Girona, Spain. E-mail: sebastia@lequia.udg.cat; Fax: +34 972418150; Tel: +34 972418182
bAbengoa Water SLU, Dos Hermanas, Sevilla, Spain
First published on 13th July 2017
Abstract
Microbial fuel cell (MFC) technology is a bio-approach to remove organic matter and nitrogen from wastewater with concomitant production of renewable electricity. Nowadays, there exists clear interest in moving MFCs towards application. This study aims to demonstrate the feasibility of MFC technology for treating swine manure. A couple of 6-stacked MFCs presenting a total volume of 115 L were designed and operated to treat swine manure at 50 L d−1 for more than 6 months. Two different electrodes were tested, one for each stacked MFC: granular graphite (GG-MFC) and stainless steel mesh (SS-MFC). Organic matter was oxidised in the anode compartments, ammonium was oxidized to nitrate in an external aerated reactor, and nitrate was reduced to dinitrogen gas in the biocathodes. GG and SS-MFCs reached similar organic matter and nitrogen removal rates (1.9 ± 0.3 kg COD m−3 d−1; 0.35 ± 0.02 kg N m−3 d−1) with power densities between 2–4 W m−3, the central units being the most electroactive. However, the GG-MFC performance declined over time due to electrode crushing and the clogging of granular graphite which reduced its applicability in comparison with stainless steel. The application of the stacked SS-MFC with a mixed electric circuit is a feasible strategy to maintain or even improve treatment efficiencies and power densities when scaling-up MFCs.
Water impactThe development of microbial fuel cells (MFCs) able to remove organic matter and nitrogen from wastewater concomitant with electricity production requires studies focused on scaled pilot plants for their future real-world implementation. In this paper, the real potential of a stacked configuration scaled-up MFC for swine manure treatment towards application was evaluated, revealing that the use of a granular graphite electrode material was not appropriate for long-term operation. |
1. Introduction
Microbial fuel cells (MFCs) are a technology to treat wastewater while recovering bioenergy.1 MFCs utilize exoelectrogenic microorganisms as catalysts to convert chemical energy of organic substrates into electricity in their anode compartment.2,3 The protons and electrons generated migrate to the cathode where reduction reactions take place (either chemically or biologically catalysed).4This technology has been widely tested to treat a great variety of pollutants including industrial wastewater in mL-scale reactors, obtaining relevant knowledge about MFC fundamentals.5–7 The ability of MFCs is not limited to the treatment of organic matter. Several authors demonstrated simultaneous treatment of multiple pollutants (i.e. carbon and nitrogen sources) using both anode and cathode compartments.8,9 MFCs could become a sustainable wastewater treatment alternative technology with potential advantages over other technologies (i.e. anaerobic digestion). Nowadays, both technologies could not be directly comparable due to their differences in the development levels, but MFCs could entail several advantages with respect to anaerobic digestion, including treatment of multiple pollutants, lower energy consumption, directly obtaining energy, smaller environmental footprint and lower sludge generation.10
The small prototypes must be scaled-up to generate enough electricity for practical applications and future implementations. However, there are some drawbacks in the practical feasibility of scaled-up MFCs, especially with respect to cost, system development and energy recovery.11 The first attempts to scale-up MFCs started a few years ago and they have been developed until today, representing 22% of the total MFC publications in the Web of Science.12 The initial problematic point was the “underdesigned” scaled-up reactors in terms of the total electrode surface area or electrode spacing. Liu et al. demonstrated that the power density could be maintained during reactor scale-up, increasing the anode surface area.13 A new challenge of BES scale-up was the reactor design, and for this reason successive studies were focused on different configurations and media employed. A 2.5 L square-MFC treating acetate was used to demonstrate the viability of the technology with 70% of the acetate removed, but achieving a low power density (2.3 W m−3 NAC) for the high ohmic cell resistance (1.4–1.7 mΩ m−3 NAC) of the MFC.14 Zhang et al. evaluated 2 L tubular-MFCs treating acetate enriched wastewater and urban wastewater with bioelectricity production. Usually, the nitrogen in wastewater is in the form of ammonium. It requires a previous oxidation step to nitrate before being removed by a MFC simultaneously with the organic matter. A couple of cathode electron acceptor (oxygen and nitrate) configurations were used to determine their effect on organic matter removal rates and power production.15,16 The organic matter removal efficiencies were maintained over 60% treating urban wastewater and current production was almost 7 times higher (15 W m−3 NAC) than in the previous study. Once nitrogen (in the form of nitrate) was incorporated into the medium, 76% of the nitrogen was removed in the cathode but the energy production substantially decreased (8 W m−3 NAC) because of the occurrence of heterotrophic denitrification of the remaining anodic organic matter.
In spite of these promising attempts, the energy recovery and the volumetric capacities in scaled-up MFC reactors with single units were insufficient. Multiple stacked MFCs started to be tested in order to improve the systems. Different electrical configurations (i.e. series or parallel) were tested in stacked MFCs to achieve higher voltage or current.17 However, the series connection can suffer from voltage reversal, contact voltage losses and erratic operation, while in the parallel connection internal losses increase, which reduces the total power production.18
The first attempts at stacked MFCs were performed on 1 L-scale MFCs, including a 12 pair cassette-electrode MFC, obtaining high organic matter removal rates (5.4 kg COD m−3 d−1) and power production (129 W m−3 NAC).19 It was reported that a MFC consisting of 4-stacked MFC reactors with a total volume of 20 L, maintained its power density (140 W m−3 NAC) with respect to mL-reactors.20 Jiang et al. also operated a stacked MFC of 16 L with urban wastewater obtaining low removal rates (0.2–1.0 kg COD m−3 d−1) with low electricity production (0.4–0.9 W m−3 NAC) due to the precipitation of calcium and sodium carbonates in the cathode which increased the internal resistance.21
The success of stacking bench-scale MFCs encouraged the scaling-up of the MFC modules towards larger scale systems. In these cases, alternative parallel/series connections were applied in order to charge and discharge the capacitors successively. As a result, a 90 L rectangular MFC reactor vessel achieved sufficient energy treating brewery wastewater that supported its operation.22 A 200 L modularized MFC system consisting of 96 tubular MFC modules was examined for long-term (one year) performance treating municipal wastewater. The MFC was able to achieve removal efficiencies over 65% for organic matter and nitrogen but the electricity production was limited to 1 W m−3 NAC.23 The biggest MFC pilot plant was constructed in Queensland (Australia) consisting of 12 tubular-MFC modules with a total liquid volume of 1 m3.24 The pilot performance was unsuccessful due to the low conductivity and high biomass proliferation due to organic matter excess.25
The applicability of the scaled-up stacked MFCs was tested working with municipal and brewery wastewater, but there are few examples focusing on the treatment of complex matrices such as swine manure on this scale. A 1.5 L 5-stacked tubular air-cathode MFC was evaluated in terms of simultaneous real swine manure treatment and bioelectricity generation. Although it showed relatively fast removal rates (between 1.0–3.2 kg COD m−3 d−1), the power density was reduced by two orders of magnitude with respect to that of the simplest wastewater matrices, 4 W m−3 NAC.26 The highest volumetric example consisted of a 3.7 L constructed wetland MFC with an aerated cathode. The achieved COD removal rate and power density were lower than the predecessor study treating swine manure.27 The low treatment and volumetric capacities of these reactors, with flows below 4 L d−1, threaten their real applicability.
This study aims to scale-up stacked MFCs for swine manure treatment. The performance of a couple of 6-stacked MFCs with different electrodes, granular graphite (GG) and stainless steel (SS), was evaluated in terms of organic matter and nitrogen removal rates and efficiencies, energy production and material life expectancy. The evolution of these compounds inside the 6 rectangular MFC units and their electrochemical behaviour were monitored in order to identify the activity differences between units. Finally, several electric connections such as series, parallel and mixed (parallel–series) were tested in order to optimise the renewable electricity production.
2. Materials and methods
2.1. Stacked MFC design
The stacked MFC was designed and operated to remove organic matter and nitrogen from swine manure with concomitant bioelectricity production. The MFCs consisted of six anodic and cathodic compartments (90 × 40 × 1.5 cm each one) hydraulically connected to an external nitrifying reactor (150 cm × 20 cm diameter). The total volume of the MFCs was 65 L while that of the external tubular reactor was 50 L (system gross capacity of 115 L). The anode and cathode compartments were placed on opposite sides of a polyvinyl chloride rectangular compartment, clamped transversely together by stainless steel bolts (Fig. 1). Moreover, they were separated by an anionic exchange membrane (AMI-7001, Membranes International Inc., USA) to avoid ammonium diffusion to the cathode.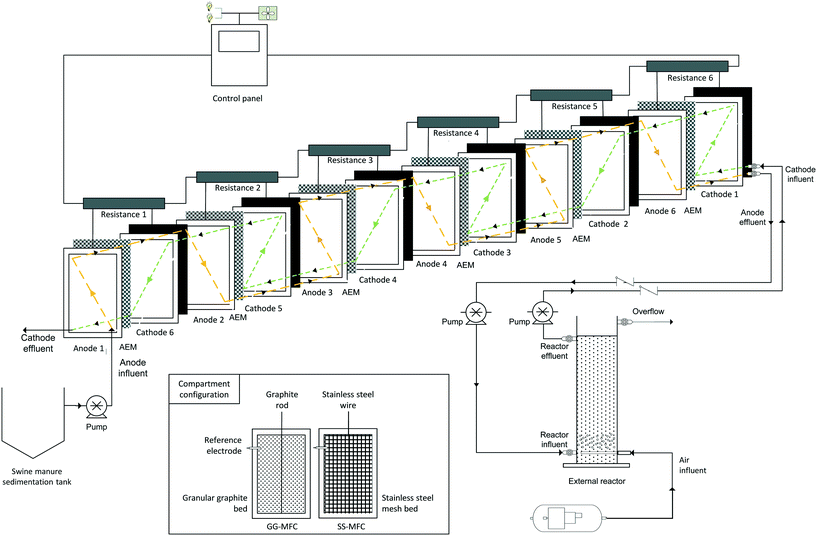 | ||
| Fig. 1 Schematic diagram of the reactor set up with coloured hydraulic fluxes of the anodes (orange) and cathodes (green). The compartment configuration is also shown, where the GG-MFC was filled with granular graphite and a graphite rod, while the SS-MFC was filled with stainless steel mesh and a stainless steel wire.28 | ||
Swine manure was stored in a sedimentation tank where the solid was pre-settled. The stacked MFC was continuously fed at a flow rate of 50 L d−1. The anodes and cathodes were connected with a counter current flux. The purpose of the system was to feed the swine manure through the anode set of compartments in order to oxidize the organic matter by exoelectrogenic bacteria. The influent was transferred from the 1st anode to the 6th anode compartment (orange flow, Fig. 1). Then, the anode effluent was used to feed the aerated external nitrifying reactor to oxidize ammonium into nitrate (nitrification) by nitrifying bacteria. Finally, the effluent of the nitrifying reactor was fed to the cathode set of compartments to reduce nitrate into dinitrogen gas (denitrification) by electrotrophic bacteria. The cathodes followed the same hydraulic strategy as the anodes but in the opposite direction, starting the treatment of nitrate from the 1st cathode and finishing at the 6th cathode (green flow in Fig. 1). The temperature was kept constant at 23 ± 2 °C.
A couple of configurations were assessed depending on the electrode material. In one of the configurations, granular graphite (model 00514, diameter 1.5 × 105 mm, EnViro-cell, Germany) was used as the electrode material (GG-MFCs) and graphite rods (120 cm × 0.6 cm) as electrode collectors (Mersen Iberica, Spain). The filling material decreased the volumes of the compartments, reaching 20 L of the net anodic and cathodic compartments (NACs and NCCs, respectively). In the other configuration, a double layer of stainless steel mesh (90 × 40 × 0.1 cm every layer, model 316 L, Cisa, Spain) was used as the electrode material (SS-MFCs) and stainless steel wires as electrode collectors. The filling material reduced the volumes to 37 L of the NACs and NCCs. All configurations had one Ag/AgCl reference electrode in each compartment (+0.197 V vs. SHE, model RE-5B, BASi, UK). The anodes and cathodes were individually connected to an external resistance of 1.5 Ω to close the electric circuit.
In both configurations, an external tubular reactor of PVC with a net reactor compartment (NRC) volume of 20 L was built to perform aerobic ammonium oxidation to nitrate (nitrification). The reactor was filled with clay (diameter 0.8 cm) to promote bacteria adhesion.9 Aeration from the bottom of the reactor was performed using an air compressor (B2800B/100 CM3 2 CIL, Ingersoll Rand, UK). Dissolved oxygen concentration was controlled and limited to values between 1–1.5 mg O2 L−1 using a dissolved oxygen probe (Model 50 60, Crison, Spain) to limit the oxygen influence on the anoxic cathodic compartment.
2.2. MFC operation
The anode compartments of the MFC reactors were inoculated with the anode effluent of activated sludge from the Girona wastewater treatment plant (WWTP) and a parent lab-scale MFC treating swine manure.9 The nitrifying reactor was inoculated with biomass from a biological treatment of the same WWTP and with activated sludge from a partial nitritation reactor treating high ammonium landfill leachate.29 The cathodes were inoculated with activated sludge and with the effluent of a parent lab-scale denitrifying bioelectrochemical system.30 The start-up period of both configurations finished after a couple of weeks working in continuous mode (50 L d−1). The continuous operation feeding with swine manure was evaluated over 6 months for each configuration (GG-MFC and SS-MFC).2.3. Swine manure
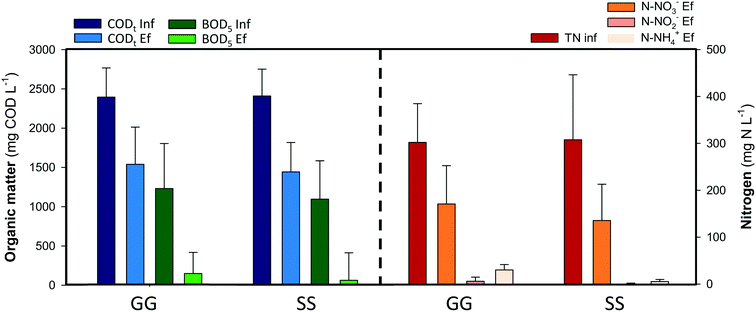
Swine manure was taken from the Food and Agricultural Research Institute (IRTA) of Monells (Spain). It was stored in a refrigerated sedimentation tank (6 °C) to promote the settling of solids and minimize degradation over time. The supernatant was fed into the 1st anode chamber of the MFCs. Table 1 presents the main characteristics of the swine manure supernatant used during the experimental period.| Swine manure | Units | |
|---|---|---|
| n.d.: not detected. | ||
| pH | 8.5 ± 0.2 | — |
| Conductivity | 8.3 ± 0.4 | mS cm−1 |
| Alkalinity | 3330 ± 900 | mg CaCO3 L−1 |
| CODTotal | 2470 ± 490 | mg COD L−1 |
| CODSoluble | 2290 ± 460 | mg COD L−1 |
| BOD5 | 1225 ± 125 | mg BOD L−1 |
| TKN | 305 ± 86 | mg TKN-N L−1 |
| NH4+ | 245 ± 50 | mg NH4+-N L−1 |
| NO2− | n.d. | mg NO2−-N L−1 |
| NO3− | n.d. | mg NO3−-N L−1 |
| N2O | n.d. | mg N2O-N L−1 |
| TSS | 1150 ± 100 | mg TSS L−1 |
2.4. Electrochemical configuration
The electrochemical connection of stacked MFCs can be arranged in a number of different ways. The electrical connections used for the 6-stacked MFC were (i) individual (normal operation), (ii) in parallel, (iii) in series and (iv) mixed (3 MFCs in parallel and 3 in series), to step-up the current, voltage or both, respectively (Fig. 2). The external resistance applied under individual MFC connection was 1.5 Ω, those in parallel connection were 15 and 100 Ω and that in series connection was 2200 Ω in order to be higher than the internal resistance found in the polarization curves. The mixed connection joined the MFCs depending on their internal resistances. MFCs 1–6, 2–3 and 4–5 were arranged in series due to their similar internal resistance. Once the MFCs were connected in series, they were arranged in parallel. The resistance applied was 100 Ω.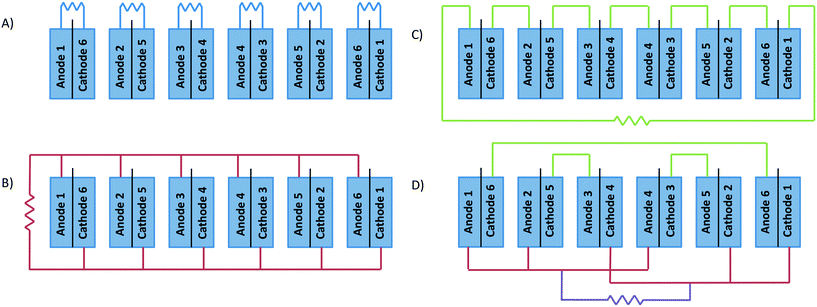 | ||
| Fig. 2 Schematic representation of the electrical circuit connection in the 6-stacked MFCs. A) individual, B) in parallel, C) in series and D) mixed (parallel–series). | ||
2.5. Chemical and electrochemical analyses and calculations
Liquid-phase standard wastewater measurements for total and soluble organic matter (CODt and CODs), 5-day total and soluble biodegradable organic matter (BOD5t and BOD5s), total and volatile suspended solids (TSS and VSS), and nitrogen (total Kjeldahl nitrogen (TKN-N), ammonium (NH4+-N), nitrite (NO2−-N) and nitrate (NO3−-N)) were performed at regular intervals according to the American Public Health Association guidelines.31 Samples were obtained from the influent (first anode and last cathode) and effluent (last anode and first cathode) sections of the BES reactors. For the analysis of every compartment, the samples were taken from a side opening of each one. The free ammonia (FA) and free nitrous acid (FNA) concentrations were calculated according to Anthonisen et al.32 Organic (ORR) and nitrogen (NRR) removal rates (kg COD m−3 NAC d−1 and kg N m−3 NCC d−1, respectively) were calculated as the difference between the influent and effluent loading rates. Anode and cathode coulombic efficiencies (CE) were calculated as in Virdis et al.33Gas samples were analysed for detecting the presence of carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O) and nitrogen (N2) gases with an Agilent 7820A GC System equipped with Washed Molecular Sieve 5A and Porapak® Q columns and a thermal conductivity detector (TCD). Nitric oxide (NO) production was considered negligible.34 Gas production rates were calculated by dividing the obtained gas volume per unit of time.
Anode and cathode potentials were monitored with Ag/AgCl reference electrodes (+0.197 V vs. SHE, model RE-5B, BASi, UK). The current (I) and power (P) were determined according to Ohm's law (I = V/R; P = I × V). Power and current densities were calculated by dividing power and current by the NAC. Polarization curves were obtained using a potentiostat (model SP50, Bio-logic, France) and by imposing a linear potential decrease of 1 mV s−1 from the open circuit voltage (OCV) to a cell voltage of 0 mV and vice versa. The electron balance in the MFC units was calculated as the ratio between the carbon and nitrogen removal concentrations (ratio of C/N) and then, compared to the stoichiometric/theoretical carbon and nitrogen ratio (2.86).
3. Results
3.1. Overall performance of stacked MFCs
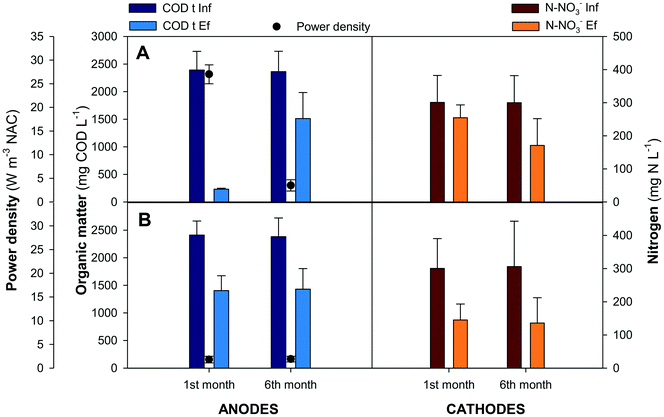
Nitrogen compounds remained invariable along the anode compartments. In the external aerated reactor more than 95% of the ammonium was oxidised to nitrate. Nitrates were removed inside the cathodes, removing 130 mg N L−1 (44 ± 10%) in the GG-MFC and 170 mg N L−1 (56 ± 15%) in the SS-MFC. The denitrifying removal rates were similar between configurations, 0.37 ± 0.1 kg N m−3 d−1 in the GG-MFC and 0.30 ± 0.1 kg N m−3 d−1 in the SS-MFC. The presence of nitrogen intermediate species in both configurations was almost negligible, less than 3% of the nitrite was accumulated, while nitrous oxide was not detected. The low cathodic CE achieved (16 ± 3% and 13 ± 2% in GG and SS MFCs, respectively) indicated an alternative process to remove nitrate (e.g. heterotrophic denitrification). In terms of energy recovered, the GG-MFC achieved slightly higher power densities (3.5 ± 1.2 W m−3 NAC) than the SS-MFC (1.9 ± 0.6 W m−3 NAC).
Granular graphite was used in the GG-MFC for 6 months. The granules touch each other and have an intrinsic low porosity of 0.53. A potential clogging effect either from bacteria growth on the electrode or particles from wastewaters could negatively affect its structure. All these effects increased the overpotentials of the cells over time with a concomitant decrease of energy production and change of the organic matter and nitrogen removal rates. The power density achieved was reduced by one order of magnitude between the beginning and the end of the experimental period, from 27 ± 2 W m−3 NAC to 3.5 ± 1.2 W m−3 NAC, as shown in Fig. 4. The organic matter removal in the anode compartments decreased from 90 ± 2% to 36 ± 7%, meanwhile, in terms of nitrate, the nitrogen removal in the cathodes increased from 15 ± 2% to 44 ± 10%. In both cases, the anodes and cathodes, the solid accumulation in the anodes results in low anode performances and high denitrification efficiencies in the cathodes, mainly due to heterotrophic denitrification. At the end of the experimental period, the granular graphite electrode was crushed (Fig. S1 and S2†).
Therefore, another material (stainless steel) with similar characteristics to granular graphite in terms of conductivity and cost was used in the other configuration (SS-MFC). Stainless steel showed similar removal efficiencies in the anodes and cathodes, avoiding problems such as clogging or electrode compaction. Moreover, the solids accumulated on the bottom of the first anode compartment in the suspension, instead of being attached to the electrode.
3.2. Unravelling the dynamics in each MFC unit
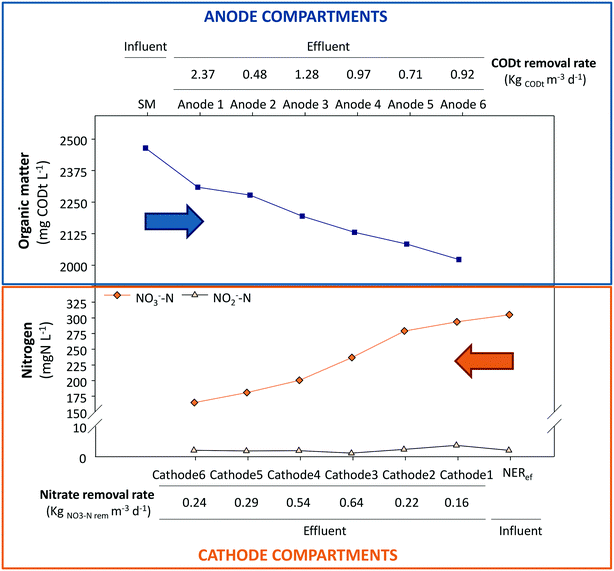
The study of the nutrient and electrical dynamics of each unit of the stacked MFC was performed with the GG-MFC configuration under steady state conditions. The influent swine manure crossed the anodes one by one and then, the cathodes in the same way but in the opposite direction, suggesting internal gradients of organic matter and nitrogen, respectively. Fig. 5 presents the evolution of the concentrations of organic matter and nitrogen compounds along the stacked MFC units.The organic matter concentration inside the anode compartment decreased gradually. The oxidation rate tendency showed high oxidation rates in the first compartments and lower at the last ones. The maximum organic matter removal rate (2.37 kg COD m−3 d−1) was obtained in the first compartment. In this case, the solids from swine manure, not decanted in the refrigerated settler tank, were partially retained in the 1st anodic compartment. From then on, the central units (anodes 3 and 4) showed higher removal rates (1.28 and 0.97 kg COD m−3 d−1, respectively) than the contiguous units. In the last anode, the oxidation rate diminished slightly to 0.92 kg COD m−3 d−1. Around 2 g L−1 COD of organic matter remained in the liquid due to the low anodic HRT applied (9.6 hours).
A different behaviour was observed for the nitrogen treatment. The central cathodic units (cathodes 3 and 4) were the units with the highest denitrifying rates (0.64 and 0.54 kg NO3−-N m−3 d−1, respectively). The lowest denitrifying rate was obtained at the 1st cathode, where the influence of the aerated external reactor could negatively influence the process. Nevertheless, nitrate was not completely reduced in the cathodes (0.17 g L−1 NO3−-N), which certainly indicates that some limitation existed for denitrifying bacteria in this system.
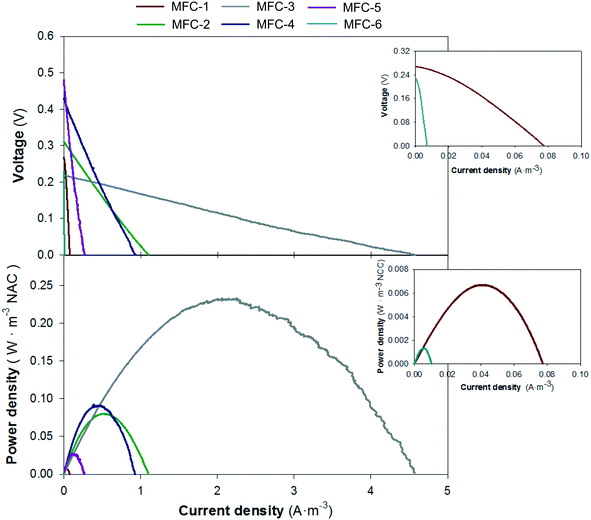
The theoretical electron balance between carbon (mg COD L−1) and nitrogen (mg N L−1) for complete removal is 2.86. Units 1 and 6 showed values far from the theoretical ratio (9.94 and 5.58, respectively), which is in line with the high organic matter removal rates in the first unit due to the solid removal effect and low denitrification removal rates in the last unit due to the influence of the external reactor. The central units had C/N values closer to 2.86 (1.63, 2.35, 1.53 and 3.23, for units 2, 3, 4 and 5, respectively) than the peripheral units, which indicated a better balanced electron flux between the anodes and cathodes. These results matched with the electrochemical performance of each individual MFC (Fig. 6) where the fastest treatment rate corresponded to unit 3 that showed the highest power density, 0.23 W m−3 NAC.
3.3. Electrochemical characterization of the stacked MFCs
Different electric circuit conditions were tested to enhance renewable electricity production. The electrical connections tested for the 6-stacked MFC were in parallel, in series and mixed (3 MFCs in parallel and 3 in series), to step-up the current, voltage or both, respectively. The parallel circuit connection under different resistances (15 and 100 Ω) showed the lowest internal resistance (<4 Ω) and the highest current densities (2–3 A m−3 NAC in both cases) among the set of electric connections tested (Fig. 7). In contrast, the series connection increased the internal resistance of the system and decreased the intensity and energy obtained. In addition, occasional problems with voltage reversal from two of the six MFCs (1st and 6th MFCs) indicated that this electrical configuration was not the ideal for this system. The mixed circuit substantially reduced the internal resistance of the stacked MFC with respect to the series circuit, and maintained the current density (1.89 A m−3) of the parallel circuit. This combination resulted in the highest power density (0.33 W m−3) achieved. Furthermore, there was no problem of voltage reversal as that observed in the series connection.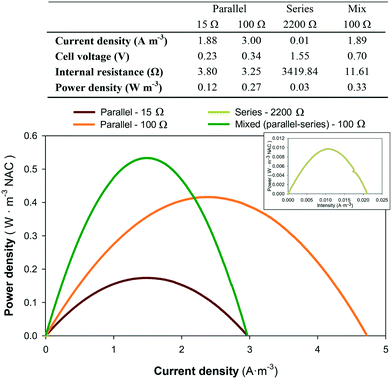 | ||
| Fig. 7 Power density curves of the stacked MFCs connected at different electric circuit connections: parallel (15 Ω and 100 Ω), series (2200 Ω) or mixed (100 Ω). | ||
The electrode clogging effect could negatively influence the removal performances, without observable significant differences in terms of organic matter and nitrogen removal concentrations and rates.
4. Discussion
4.1. Assessment of the electrode material for 6-stacked MFCs
The performance of the 6-stacked MFCs with different electrode materials was evaluated and compared with previous research reports using synthetic, urban or industrial medium as the influent (Table 2). In this study, more than 90% of the BOD (1.9 ± 0.3 kg COD m−3 d−1 removal rate; CEs of 17 ± 4%) and 50% of the total nitrogen content (0.35 ± 0.02 kg N m−3 d−1 removal rate; CEs of 15 ± 2%) were removed, achieving power densities between 2–4 W m−3 in both configurations. The COD removal rates and CEs achieved were below the ranges reported using synthetic media, where the removal rates ranged between 2.5–5.5 kg COD m−3 d−1 with corresponding CEs of 48% and 28%.19 Real wastewater influents showed lower removal rates and CEs with respect to synthetic media, achieving values between 0.1–1.0 kg COD m−3 d−1 with CEs generally lower than 10%.21–23 Several authors related it to the toxic effect of intermediate product accumulation on the complex reactions taking place, which reduces the organic matter removal efficiency, and to its highly complex nature that promotes side reactions such as fermentation and/or methanogenesis and other biological processes, reducing the CEs achieved.36,37| Influent wastewater | Configuration | Net volume (L) | Organic matter removal | Nitrogen removal | Power density (W m−3) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OLR (kg COD m−3 d−1) | ORR (kg COD m−3 d−1) | ORE (%) | Anode CE (%) | NLR (kg N m−3 d−1) | NRR (kg N m−3 d−1) | NRE (%) | Cathode CE (%) | |||||
| Synthetic wastewater | 12-Chamber cassette MFC | 1 | 2.90 | 2.70 | 95 | 48 | No nitrogen treatment performed | 117 | 19 | |||
| 5.80 | 5.39 | 93 | 28 | 129 | ||||||||
| Brewery wastewater | 5-Stacked dual chamber MFC | 90 | 0.16 | 0.14 | 88 | 8 | No nitrogen treatment performed | 97 | 22 | |||
| 0.27 | 0.23 | 85 | 19 | 56 | ||||||||
| Urban wastewater | 12-Stacked dual chamber MFC | 16 | 0.24 | 0.21 | 88 | <1 | 0.05 | 0.02 | 30 | n.d. | <1 | 21 |
| 1.08 | 0.99 | 92 | <1 | 0.01 | 0.01 | 30 | n.d. | <1 | ||||
| 96-Stacked dual chamber MFC | 200 | 0.31 | 0.12 | 38 | n.d. | 0.05 | 0.03 | 68 | n.d. | 8 | 23 | |
| Swine manure | Single wetland MFC | 3.7 | 0.83 | 0.19 | 77 | <1 | No nitrogen treatment performed | <1 | 27 | |||
| 5-Stacked single chamber MFC | 1.5 | 1.20 | 0.99 | 83 | <1 | 0.11 | 0.09 | 87 | n.d. | 4 | 26 | |
| 4.90 | 3.23 | 66 | <1 | 0.52 | 0.41 | 80 | n.d. | n.d. | ||||
| 6-Stacked dual chamber GG-MFC | 60 | 5.00 ± 0.50 | 2.10 ± 0.50 | 36 ± 7 | 17 ± 3 | 0.75 ± 0.30 | 0.37 ± 0.10 | 44 ± 10 | 16 ± 3 | 4 ± 1 | This study | |
| 6-Stacked dual chamber SS-MFC | 94 | 5.00 ± 0.50 | 1.60 ± 0.70 | 40 ± 15 | 17 ± 4 | 0.41 ± 0.10 | 0.30 ± 0.10 | 56 ± 15 | 13 ± 2 | 2 ± 0 | This study | |
No scaled-up BESs over 4 L for swine manure treatment have been reported in the literature. A 1.5 L 5-stacked tubular MFC working at a similar OLR was able to achieve higher organic rates than the ones presented in this study (3.23 kg COD m−3 d−1; 66% of COD removed) but achieved CEs lower than 0.2%. These results indicated an almost non-electrogenic treatment of the organic matter. Once the OLR was reduced, the treatment efficiencies increased (83% COD removal) but the removal rate was reduced to 0.99 kg COD m−3 d−1 and the CEs did not improve. This effect was also demonstrated on the mL-scale with other wastewaters, obtaining higher treatment efficiencies (over 80%) when the OLR decreased.38,39 In these cases, the lower OLR corresponded with longer HRTs that allowed more exposure time of organic matter to bacteria, increasing its degradation.40,41 The biggest BES tested was a constructed wetland MFC of 3.7 L. On increasing the volumetric capacity, the achieved COD removal rate was reduced to 0.19 kg COD m−3 d−1 and the CE was maintained at values lower than 1% (77% COD removal efficiency) at a low OLR of 0.83 kg COD m−3 d−1.27 This result was in accordance with the tendency of all scale-up reactors, independent from the liquid source.21,23 The 6-stacked MFC evaluated in this study allowed the increase of the net volume reactor between 16 and 25 times with respect to the biggest MFC designed to treat swine manure, achieving similar removal rates to the 1.5 L MFC.26,27 Moreover, the configuration used in this study was able to establish and maintain the electrogenic process for a long-term.
BESs were proposed as a new alternative for nitrogen treatment in swine manure waste due to the lack of elimination of this compound in anaerobic digestion.42 The development of a sustainable and robust system for nitrogen removal is still missing. No BES scale-up attempt for nitrogen treatment has been reported until this study. Similar nitrifying efficiencies (over 90% in both cases) and denitrifying removal rates (0.37 ± 0.1 kg N m−3 d−1 in the GG-MFC and 0.30 ± 0.1 kg N m−3 d−1 in the SS-MFC) were achieved between the electrode materials, with cathodic CEs of 16 ± 3% and 13 ± 2% in the GG and SS MFCs, respectively. In terms of nitrogen, the configuration showed in this study was already tested on the mL-scale.9,33,43 These studies were able to achieve similar efficiencies for ammonium oxidation (>90%) with respect to the ones presented in this study, while lower nitrate reduction rates were achieved (0.40, 0.01 and 0.16 kg N m−3 d−1, respectively, with corresponding CEs of 70–80%, 20% and 10%). The higher removal efficiencies corresponded to higher HRTs (1–5 d) and lower nitrogen concentrations (40 mg N L−1). The low CE achieved indicated an alternative process to remove nitrate. In the present study, the migration of nitrates to the anode through the membrane and heterotrophic nitrate removal could explain it. However, nitrogen species, such as nitrate or nitrite, were not detected in the anode compartments. These results suggested that bioelectrochemical nitrate reduction is the limiting step for nitrogen treatment from swine manure using BESs.
In terms of energy recovered, the GG-MFC achieved a power density of 4 ± 1 W m−3 while the SS-MFC achieved 2 ± 1 W m−3. The results obtained were in agreement with those of the other stacked MFC treating swine manure, which obtained a power density of 4 W m−3 even if usually lower values were obtained when treating swine manure (0.02 W m−3 in Zhao et al.27) and urban wastewater (0.35–0.90 W m−3 in Jiang et al.21).26 The studied stacked MFC allowed the maintenance of the removal rates and the power output achieved for the mL-scale stacked MFCs treating complex wastewater matrices.
In all BESs scaled-up to treat swine manure, a carbon electrode was used as the electrode material bed, either for the anode and cathode compartments.26,27 A couple of electrodes (GG-MFC and SS-MFC) were evaluated in the present study for organic matter and nitrogen treatment, obtaining similar removal rates and electricity generation. The results suggested that the granular graphite electrode material was not appropriate for long-term operation for any compartment (anodes and cathodes), obtaining graphite blocks that did not allow the appropriate liquid distribution inside the compartment. Moreover, the presence of solids (1.2 ± 0.1 g TSS L−1) caused packing of the anode compartments and it could also negatively influence the membrane functionality. With respect to the stainless steel electrode material, the high corrosion risk could compromise its applicability in scaled-up MFCs. This effect was not appreciated in the six anodes used in this study. The appropriate operational conditions applied44 and potentials lower than its standard oxidation potential (−0.21 vs. SHE at pH 7)45,46 in the anodes reduced the material corrosion. Meanwhile, the reductive nature of the cathodes protected the stainless steel against corrosion, making it a good option for long-term treatment of wastewaters.
4.2. Performance of individual MFC units
The study of the nutrient and electrical dynamics of each individual unit of the stacked MFC concluded that the central units were the most electro-active compartments, which had the highest organic matter and nitrogen removal rates (1.28 and 0.97 kg COD m−3 d−1; 0.64 and 0.54 kg NO3−-N m−3 d−1) and power densities (0.23 and 0.09 W m−3 NAC). These results agreed with the electron balance performed between the anode and cathodes which were near the stoichiometric carbon/nitrogen ratio (central units), indicating that, apparently, no electron limitation existed when carrying out the two bioelectrochemical processes (carbon oxidation and nitrate reduction). In contrast, the high organic matter removal rate of the extreme anode compartments (peripheral units) could be explained by the high complexity of organic matter and solid retention. The nitrogen treatment in the cathodes was limited by the dissolved oxygen in the liquid phase coming from the external reactor. In general, nitrate bioelectrochemical removal was limited, since nitrate was not completely reduced from the cathodes. The operational HRT applied as well as the intrinsic overpotentials (reactor size, reactor configuration, etc.) could have played a role in this limitation. Zhuang et al. demonstrated that complete removal efficiencies could be achieved by increasing the HRT but this negatively affects the power density generated.26 Other authors who used a similar hydraulic fluid reactor observed different performances in terms of organic matter treatment and energy production between the stacked MFC units with respect to the ones observed in this study.5,26,47 Their studies support that the most electroactive compartment is the last one, where the substrate is more accessible. In our configuration, the solid retention and exhaustion of the BOD in the anodes, the low HRT and the influence of the external reactor on the cathodes made the central unit the most electroactive.4.3. Electric configuration optimization
Several scaled-up reactors were electrically configured in parallel or in series circuits, achieving the highest current and power densities for their systems, respectively.17,18,26,47 However, the mixed circuit substantially lowered the internal resistance of the stacked MFC, leading to a higher power density (0.33 W m−3) at a relatively higher current density (1.89 A m−3). Furthermore, there was no problem of voltage reversal as that observed in the series connection. The high number of parallel connections applied in the mixed circuit greatly enhanced the power and current densities achieved, as demonstrated by Papaharalabos et al.48 Consequently, the mixed circuit was considered the most appropriate connection due to its high applicability. The maximum power density achieved with this circuit will consequently reduce the number of units required for the other electric circuits to obtain the same power output while the high current densities will allow the better workability of the cathodic reaction, and thus a higher denitrification rate might be expected.Although in this study external resistances were applied in order to minimise the operational cost, energy could not be harvested. For this reason, the development of power management systems to harvest energy, shaping it to a usable form, could be considered.49 Nowadays, integrated circuits or chips are commonly used in several electronic devices due to their small volume, low cost, low energy consumption, and quick switch among components.49 These characteristics made them good candidates for application in MFCs. The first attempts already achieved high efficiencies in terms of energy harvested in MFCs,50,51 however, more research is necessary in order to reduce the energy demand of these additional systems and to self-sustain the operation of the MFC reactors.
5. Conclusions
The results provided a better understanding of the real potential of MFCs, highlighting the challenges of MFC scaling-up for swine manure treatment towards application. The low HRT applied (9.6 hours in anodes and cathodes) did not allow the complete degradation of the organic matter and nitrogen compounds, but the biodegradable fraction was almost completely consumed. High organic matter and nitrogen removal rates of 2.1 ± 0.5 and 1.6 ± 0.7 kg COD m−3 d−1, and 0.4 ± 0.1 and 0.3 ± 0.1 kg N m−3 d−1 for GG and SS-MFCs, respectively, were achieved. In both cases, anodic and cathodic electrogenic activities were around 15%, achieving power densities of 4 ± 1 and 2 ± 0 W m−3 NAC for GG and SS-MFCs, respectively. The values were considered high due to the complexity of the organic matter in swine manure, which could have promoted side reactions. The clogging effect of the granular graphite bed used in the GG-MFC reduced its applicability for long-term operation. The stainless steel used in the SS-MFC should be considered as a promising material for scaled-up reactors due to its easy applicability, cheap price, high treatment efficiencies, and lack of clogging effect and corrosion problems. The treatment capacity of the different units showed that the central units were the most electroactive in the stacked MFC. Moreover, the utilization of a mixed electric circuit between units increased the power and current density achieved. The application of this configuration could be a feasible strategy to maintain or even improve treatment efficiencies and power densities when scaling-up MFCs towards application.Acknowledgements
The authors wish to acknowledge the assistance of Ms. Dorine Glautier, Mr. Albert Vilà-Rovira, Dr. Narcis Pous and Dr. Pau Batlle-Vilanova for their support on the reactor design, construction and operation. This research was financially supported by the Company Abengoa Water within the TEcoAgua project (CIEN-20091028), the Spanish Government (CTQ2014-53718-R) and the Catalan Government (2014 FI-B 00093). LEQUIA has been recognised as consolidated research groups by the Generalitat de Catalunya with code 2014-SGR-1168.References
- B. E. Logan and K. Rabaey, Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies, Science, 2012, 337(6095), 686–690 CrossRef CAS PubMed.
- D. R. Lovley, The microbe electric: conversion of organic matter to electricity, Curr. Opin. Biotechnol., 2008, 19(6), 564–571 CrossRef CAS PubMed.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Microbial fuel cells: methodology and technology, Environ. Sci. Technol., 2006, 40(17), 5181–5192 CrossRef CAS PubMed.
- D. R. Lovley, Powering microbes with electricity: direct electron transfer from electrodes to microbes, Environ. Microbiol. Rep., 2011, 3(1), 27–35 CrossRef CAS PubMed.
- L. Zhuang, Y. Yuan, Y. Wang and S. Zhou, Long-term evaluation of a 10-liter serpentine-type microbial fuel cell stack treating brewery wastewater, Bioresour. Technol., 2012, 123, 406–412 CrossRef CAS PubMed.
- S. Puig, M. Serra, A. Vilar-Sanz, M. Cabré, L. Bañeras, J. Colprim and M. D. Balaguer, Autotrophic nitrite removal in the cathode of microbial fuel cells, Bioresour. Technol., 2011, 102(6), 4462–4467 CrossRef CAS PubMed.
- S. B. Velasquez-Orta, I. M. Head, T. P. Curtis and K. Scott, Factors affecting current production in microbial fuel cells using different industrial wastewaters, Bioresour. Technol., 2011, 102(8), 5105–5112 CrossRef CAS PubMed.
- A. Hussain, M. Manuel and B. Tartakovsky, A comparison of simultaneous organic carbon and nitrogen removal in microbial fuel cells and microbial electrolysis cells, J. Environ. Manage., 2016, 173, 23–33 CrossRef CAS PubMed.
- A. Vilajeliu-Pons, S. Puig, N. Pous, I. Salcedo-Dávila, L. Bañeras, M. D. Balaguer and J. Colprim, Microbiome characterization of MFCs used for the treatment of swine manure, J. Hazard. Mater., 2015, 288, 60–68 CrossRef CAS PubMed.
- K. Rabaey and W. Verstraete, Microbial fuel cells: novel biotechnology for energy generation, Trends Biotechnol., 2005, 23(6), 291–298 CrossRef CAS PubMed.
- W.-W. Li, H.-Q. Yu and Z. He, Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies, Energy Environ. Sci., 2014, 7(3), 911 CAS.
- E. G. Ferreira Mercuri, A. Y. Jakubiak Kumata, E. B. Amaral and J. R. Simões Vitule, Energy by Microbial Fuel Cells: Scientometric global synthesis and challenges, Renewable Sustainable Energy Rev., 2016, 65, 832–840 CrossRef.
- H. Liu, S. Cheng, L. Huang and B. E. Logan, Scale-up of membrane-free single-chamber microbial fuel cells, J. Power Sources, 2008, 179(1), 274–279 CrossRef CAS.
- P. Clauwaert, S. Mulenga, P. Aelterman and W. Verstraete, Litre-scale microbial fuel cells operated in a complete loop, Appl. Microbiol. Biotechnol., 2009, 83(2), 241–247 CrossRef CAS PubMed.
- F. Zhang, Z. Ge, J. Grimaud, J. Hurst and Z. He, Long-Term Performance of Liter-Scale Microbial Fuel Cells Treating Primary Effluent Installed in a Municipal Wastewater Treatment Facility, Environ. Sci. Technol., 2013, 47, 4941–4948 CrossRef CAS PubMed.
- F. Zhang, K. S. Jacobson, P. Torres and Z. He, Effects of anolyte recirculation rates and catholytes on electricity generation in a litre-scale upflow microbial fuel cell, Energy Environ. Sci., 2010, 3(9), 1347 CAS.
- P. Aelterman, K. Rabaey, H. T. Pham, N. Boon and W. Verstraete, Continuous electricity generation at high voltages and currents using stacked microbial fuel cells, Environ. Sci. Technol., 2006, 40(10), 3388–3394 CrossRef CAS PubMed.
- S.-E. Oh and B. E. Logan, Voltage reversal during microbial fuel cell stack operation, J. Power Sources, 2007, 167(1), 11–17 CrossRef CAS.
- T. Shimoyama, S. Komukai, A. Yamazawa, Y. Ueno, B. E. Logan and K. Watanabe, Electricity generation from model organic wastewater in a cassette-electrode microbial fuel cell, Appl. Microbiol. Biotechnol., 2008, 80(2), 325–330 CrossRef CAS PubMed.
- A. Dekker, A. Ter Heijne, M. Saakes, H. V. M. Hamelers and C. J. N. Buisman, Analysis and improvement of a scaled-up and stacked microbial fuel cell, Environ. Sci. Technol., 2009, 43(23), 9038–9042 CrossRef CAS PubMed.
- D. Jiang, M. Curtis, E. Troop, K. Scheible, J. McGrath, B. Hu, S. Suib, D. Raymond and B. Li, A pilot-scale study on utilizing multi-anode/cathode microbial fuel cells (MAC MFCs) to enhance the power production in wastewater treatment, Int. J. Hydrogen Energy, 2011, 36(1), 876–884 CrossRef CAS.
- Y. Dong, Y. Qu, W. He, Y. Du, J. Liu, X. Han and Y. Feng, A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode, Bioresour. Technol., 2015, 195, 66–72 CrossRef CAS PubMed.
- Z. Ge and Z. He, Long-term Performance of a 200-Liter Modularized Microbial Fuel Cell System Treating Municipal Wastewater: Treatment, Energy, and Cost, Environ. Sci.: Water Res. Technol., 2016, 2(540), 1–2 Search PubMed.
- K. Rabaey, P. Clauwaert, P. Aelterman and W. Verstraete, Tubular microbial fuel cells for efficient electricity generation, Environ. Sci. Technol., 2005, 39(20), 8077–8082 CrossRef CAS PubMed.
- B. E. Logan, Scaling up microbial fuel cells and other bioelectrochemical systems, Appl. Microbiol. Biotechnol., 2010, 85(6), 1665–1671 CrossRef CAS PubMed.
- L. Zhuang, Y. Zheng, S. Zhou, Y. Yuan, H. Yuan and Y. Chen, Scalable microbial fuel cell (MFC) stack for continuous real wastewater treatment, Bioresour. Technol., 2012, 106, 82–88 CrossRef CAS PubMed.
- Y. Zhao, S. Collum, M. Phelan, T. Goodbody, L. Doherty and Y. Hu, Preliminary investigation of constructed wetland incorporating microbial fuel cell: Batch and continuous flow trials, Chem. Eng. J., 2013, 229, 364–370 CrossRef CAS.
- S. L. Abengoa Water, Sistema bioelectroquímico y procedimiento para la eliminación de materia orgánica y compuestos nitrogenados de aguas residuales, ES Pat., ES2015/070251, 2015 Search PubMed.
- J. Gabarró, R. Ganigué, F. Gich, M. Ruscalleda, M. D. Balaguer and J. Colprim, Effect of temperature on AOB activity of a partial nitritation SBR treating landfill leachate with extremely high nitrogen concentration, Bioresour. Technol., 2012, 126, 283–289 CrossRef PubMed.
- N. Pous, S. Puig, M. Coma, M. D. Balaguer and J. Colprim, Bioremediation of nitrate-polluted groundwater in a microbial fuel cell, J. Chem. Technol. Biotechnol., 2013, 88(9), 1690–1696 CrossRef CAS.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, USA, 19th edn, 2005 Search PubMed.
- A. C. Anthonisen, R. C. Loehr, T. B. S. Prakasan and E. G. Shinath, Inhibition of nitrification by ammonia and nitrous acid, J. - Water Pollut. Control Fed., 1976, 48, 835–851 CAS.
- B. Virdis, K. Rabaey, Z. Yuan and J. Keller, Microbial fuel cells for simultaneous carbon and nitrogen removal, Water Res., 2008, 42(12), 3013–3024 CrossRef CAS PubMed.
- B. Virdis, K. Rabaey, Z. Yuan, R. A. Rozendal and J. Keller, Electron fluxes in a microbial fuel cell performing carbon and nitrogen removal, Environ. Sci. Technol., 2009, 43(13), 5144–5149 CrossRef CAS PubMed.
- A. Vilà-Rovira, S. Puig, M. D. Balaguer and J. Colprim, Anode hydrodynamics in Bioelectrochemical Systems, RSC Adv., 2015, 5, 78994–79000 RSC.
- M. Behera and M. M. Ghangrekar, Performance of microbial fuel cell in response to change in sludge loading rate at different anodic feed pH, Bioresour. Technol., 2009, 100(21), 5114–5121 CrossRef CAS PubMed.
- K. R. Reddy, R. Khaleel and M. R. Overcash, Behavior and Transport of Microbial Pathogens and Indicator Organisms in Soils Treated with Organic Wastes, J. Environ. Qual., 1981, 10(3), 255–266 CrossRef.
- J. R. Kim, G. C. Premier, F. R. Hawkes, J. Rodríguez, R. M. Dinsdale and A. J. Guwy, Modular tubular microbial fuel cells for energy recovery during sucrose wastewater treatment at low organic loading rate, Bioresour. Technol., 2010, 101(4), 1190–1198 CrossRef CAS PubMed.
- A. Gálvez, J. Greenman and I. Ieropoulos, Landfill leachate treatment with microbial fuel cells; scale-up through plurality, Bioresour. Technol., 2009, 100(21), 5085–5091 CrossRef PubMed.
- L. Wei, Z. Yuan, M. Cui, H. Han and J. Shen, Study on electricity-generation characteristic of two-chambered microbial fuel cell in continuous flow mode, Int. J. Hydrogen Energy, 2012, 37(1), 1067–1073 CrossRef CAS.
- Q. Wen, Y. Wu, L. Zhao, Q. Sun and F. Kong, Electricity generation and brewery wastewater treatment from sequential anode-cathode microbial fuel cell, J. Zhejiang Univ., Sci., B, 2010, 11(2), 87–93 CrossRef CAS PubMed.
- J. B. Holm-Nielsen, T. Al Seadi and P. Oleskowicz-Popiel, The future of anaerobic digestion and biogas utilization, Bioresour. Technol., 2009, 100(22), 5478–5484 CrossRef CAS PubMed.
- F. Zhang and Z. He, Simultaneous nitrification and denitrification with electricity generation in dual-cathode microbial fuel cells, J. Chem. Technol. Biotechnol., 2012, 87(1), 153–159 CrossRef CAS.
- E. Guerrini, P. Cristiani, M. Grattieri, C. Santoro, B. Li and S. Trasatti, Electrochemical behavior of stainless steel anodes in membraneless microbial fuel cells, J. Electrochem. Soc., 2014, 161(3), H62–H67 CrossRef CAS.
- P. Ledezma, B. C. Donose, S. Freguia and J. Keller, Oxidised stainless steel: a very effective electrode material for microbial fuel cell bioanodes but at high risk of corrosion, Electrochim. Acta, 2015, 158, 356–360 CrossRef CAS.
- A. Baudler, I. Schmidt, M. Langner, A. Greiner and U. Schröder, Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems, Energy Environ. Sci., 2015, 8(7), 2048–2055 CAS.
- S. Wu, H. Li, X. Zhou, P. Liang, X. Zhang, Y. Jiang and X. Huang, A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment, Water Res., 2016, 98, 396–403 CrossRef CAS PubMed.
- G. Papaharalabos, J. Greenman, A. Stinchcombe, I. Horsfield, C. Melhuish and I. Ieropoulos, Dynamic electrical reconfiguration for improved capacitor charging in microbial fuel cell stacks, J. Power Sources, 2014, 272, 34–38 CrossRef CAS.
- H. Wang, J. Park and Z. J. Ren, Practical Energy Harvesting for Microbial Fuel Cells: A Review, Environ. Sci. Technol., 2015, 49, 3267–3277 CrossRef CAS PubMed.
- X. Zhang, H. Ren, S. Pyo, J.-I. Lee, J. Kim and J. Chae, A High-Efficiency DC-DC Boost Converter for a Miniaturized Microbial Fuel Cell, IEEE Trans. Power Electron., 2015, 30(4), 2041–2049 CrossRef.
- C. Erbay, S. Carreon-Bautista, E. Sanchez-Sinencio and A. Han, High Performance Monolithic Power Management System with Dynamic Maximum Power Point Tracking for Microbial Fuel Cells, Environ. Sci. Technol., 2014, 48(23), 13992–13999 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ew00079k |
| This journal is © The Royal Society of Chemistry 2017 |