Transgenerational toxicity of nanopolystyrene particles in the range of μg L−1 in the nematode Caenorhabditis elegans
Abstract
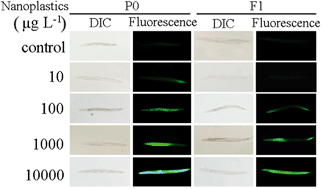
The potential toxicity of nanoplastics to environmental organisms has gradually received great attention recently. We employed the in vivo assay system of Caenorhabditis elegans to investigate the possible transgenerational toxicity of nanopolystyrene particles and the underlying cellular mechanisms. After prolonged exposure, we observed the toxicity of nanopolystyrene particles at concentrations higher than 10 μg L−1. The transgenerational toxicity was further detected in nematodes exposed to nanopolystyrene particles at concentrations higher than 100 μg L−1. This observed transgenerational toxicity of nanopolystyrene particles might be mainly due to the translocation of nanopolystyrene particles into reproductive organs such as the gonad, which potentially in turn led to the transfer of nanopolystyrene particles to the next generation. Leachates from nanopolystyrene particles at concentrations in the range of μg L−1 did not contribute to the development of this transgenerational toxicity. Enhancement of intestinal permeability and extension of defecation cycle length provide the explanation for the observed accumulation and translocation of nanopolystyrene particles in reproductive organs. Therefore, our results demonstrate the potential transgenerational toxicity of nanopolystyrene particles in the range of μg L−1 in environmental organisms.


 Please wait while we load your content...
Please wait while we load your content...