Open Access Article
Open Access ArticleEffect of surfactants, pH and water hardness on the surface properties and agglomeration behavior of engineered TiO2 nanoparticles
Frédéric
Loosli
* and
Serge
Stoll
*
Group of Environmental Physical Chemistry, F.-A. Forel Institute, Section des Sciences de la Terre et de l'Environnement, University of Geneva, 66 boulevard Carl-Vogt, CH-1211 Genève 4, Switzerland. E-mail: looslifred@gmail.com; serge.stoll@unige.ch; Fax: +41 22 379 0302; Tel: +41 22 379 0341 Tel: +41 22 379 0333
First published on 7th November 2016
Abstract
The influence of sodium dodecyl sulfate (SDS) on the stability of TiO2 engineered nanoparticle (ENP) dispersions is investigated under various pH conditions, SDS and divalent cation concentrations. Based on different scenarios and systematic measurements of surface charges and z-average sizes, a detailed mechanistic approach is proposed assuming surfactant adsorption/desorption, charge inversion, cation bridging, specific adsorption, hydrophobic effects, agglomeration and disagglomeration. Adsorption of SDS on oppositely charged TiO2 nanoparticles is found to strongly modify their stability. Formation of large agglomerates can be achieved via several routes: i) in the absence of SDS and by adjusting the pH close to the TiO2 point of zero charge, and ii) in the presence of SDS at a concentration where the positive surface charges of the TiO2 nanoparticles are counterbalanced by the SDS negative charges (charge neutralization). It is also found that hydrophobic interaction mechanisms between the SDS molecules can also promote the formation of large structures. The influence of pH variations on TiO2–SDS electrostatic complexes, formed at low pH, indicates that an excess of SDS is required to prevent the formation of large agglomerates upon pH changes. At low or intermediate SDS concentrations, TiO2 stability is governed by the subtle interplay of SDS adsorption and TiO2 surface charge acid–base properties. The presence of divalent electrolytes (water hardness) is found to reduce the SDS amount adsorbed on TiO2 ENPs and to promote the formation of large micron-sized agglomerates by cation bridging. Our results also indicate that the dispersion preparation protocol is an important issue to consider when ENPs and SDS mixtures have to be prepared and that for the formation of “individually” coated and stable nanoparticles, punctual addition of SDS is required at concentrations higher than the isoelectric point.
Environmental significanceRelatively little attention has been paid to date to the impact of surfactant molecules on the stability of manufactured nanoparticles in aquatic systems and the detailed mechanistic approach based on different scenarios and systematic measurements. In this study we demonstrate that surfactant molecules can significantly influence the TiO2 nanoparticle stability through several processes including surface adsorption and agglomeration. These processes are found reversible (desorption, disagglomeration) by changing the pH or SDS concentration. The surfactant concentration, mixing procedure (successive or punctual addition), pH as well as the presence of divalent electrolytes are also found to be of high importance. Stabilization/destabilization processes are shown here to be governed by complex mechanisms such as electrostatic repulsions, hydrophobic interactions between SDS chains, specific adsorption of divalent electrolytes, adsorption/desorption of surfactant molecules, and cation bridging. It should be also noted that for a given SDS concentration, electrostatic TiO2–SDS complexes can undergo important transformation with pH. We believe that this study is of high value for i) the establishment of ENP dispersion protocols to improve nanoparticle stability with the use of surfactant molecules, ii) a better understanding of ENP commercial dispersions stabilized by surfactants in contact with environmental matrices, iii) the understanding of the impact of water hardness on the SDS–TiO2 nanoparticle interactions in particular by considering the competition between the divalent cations and the surfactant molecules and iv) for risk assessment evaluation of ENPs entering aquatic systems where both changes in pH and water hardness are expected. |
1. Introduction
Due to their very unique properties,1 mainly arising from their huge specific surface area in comparison with bulk materials, engineered nanoparticles (ENPs) are nowadays used not only in many domains such as biomedicine, textile and cosmetic industries but also in the development of sensors and electronic devices.2–4 ENPs are produced in continuously increasing quantities to meet the needs of industry.5,6 One of the most produced nanomaterials, with more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year,7 is TiO2 due to its numerous applications and high stability.2,8–12 The stability of ENPs, which is often required for industrial applications, is strongly dependent on water chemistry such as pH and ionic strength13–15 and the presence of various compounds in the dispersion media.16–18 An important group of chemicals that are often used to stabilize ENPs during synthesis are surfactants.19–21 These amphiphilic compounds have the ability to self-assemble into well-defined structures, modify interfaces, improve dispersion stability and are frequently used for the synthesis of many inorganic nanomaterials.22,23 Surfactants are found in detergents, personal care, washing and cleaning agents as well as in numerous technical applications such as textile auxiliaries, agrochemicals, the metal and mining industry, the plastic industry and lubricants.24 According to the European surfactant producers statistics the total quantity of surfactants produced in 2013 in Western Europe was 2.9 million tons.25 Surfactants are also used as efficient compounds for the removal of heavy metals present in water via surfactant-based separation processes such as micellar-enhanced ultrafiltration.26,27 Only a few systematic studies were made to evaluate in a systematic way the stability of ENPs in the presence of surfactants. The role of the surfactant on the stability of TiO2 particles was first investigated by Imae et al.28 They studied the interaction between surfactants at concentrations above the CMC and TiO2 ENPs using absorbance, dynamic light scattering measurements and electron microscope observations. They attributed the change of absorbance in the presence of the surfactant to different adsorption modes of hemimicelles or double layer compression depending on the TiO2 surface charge pH dependence and showed that the presence of trivalent cations strongly influences the TiO2 stability and induces an arrangement of the double-layer compression. Arnold et al. investigated the adsorption of ionic surfactants on hydrophobic particles.29 This study provided a comparison of surfactant micellization in solution and agglomerate formation at one interface by considering hydrophobic and electrostatic interactions. Liu et al. investigated the stability of nano-SiO2 in the presence of a cationic surfactant30 and demonstrated that different mechanisms of ENP agglomeration (charge neutralization, depletion flocculation and hydrophobic effects) were involved in the ENP destabilization. Coagulation–flotation processes of inorganic ENPs in the presence of cationic surfactants were also investigated to optimize the removal of ENPs through particle agglomeration for the treatment of wastewaters from industries producing silica ENPs.31–33 Other studies dealing with ENP–surfactant interactions investigated the structures formed during the complexation processes between bare (or polymer coated) ENPs and surfactants by using Fourier transform infrared spectroscopy,34 small-angle neutron scattering35–37 and solvent relaxation nuclear magnetic resonance.38 In these studies, the authors observed that the presence of surfactants promoted the desorption of the polymer coating due to the conformational change of the polymer layer to a more diffuse state.
000 tons per year,7 is TiO2 due to its numerous applications and high stability.2,8–12 The stability of ENPs, which is often required for industrial applications, is strongly dependent on water chemistry such as pH and ionic strength13–15 and the presence of various compounds in the dispersion media.16–18 An important group of chemicals that are often used to stabilize ENPs during synthesis are surfactants.19–21 These amphiphilic compounds have the ability to self-assemble into well-defined structures, modify interfaces, improve dispersion stability and are frequently used for the synthesis of many inorganic nanomaterials.22,23 Surfactants are found in detergents, personal care, washing and cleaning agents as well as in numerous technical applications such as textile auxiliaries, agrochemicals, the metal and mining industry, the plastic industry and lubricants.24 According to the European surfactant producers statistics the total quantity of surfactants produced in 2013 in Western Europe was 2.9 million tons.25 Surfactants are also used as efficient compounds for the removal of heavy metals present in water via surfactant-based separation processes such as micellar-enhanced ultrafiltration.26,27 Only a few systematic studies were made to evaluate in a systematic way the stability of ENPs in the presence of surfactants. The role of the surfactant on the stability of TiO2 particles was first investigated by Imae et al.28 They studied the interaction between surfactants at concentrations above the CMC and TiO2 ENPs using absorbance, dynamic light scattering measurements and electron microscope observations. They attributed the change of absorbance in the presence of the surfactant to different adsorption modes of hemimicelles or double layer compression depending on the TiO2 surface charge pH dependence and showed that the presence of trivalent cations strongly influences the TiO2 stability and induces an arrangement of the double-layer compression. Arnold et al. investigated the adsorption of ionic surfactants on hydrophobic particles.29 This study provided a comparison of surfactant micellization in solution and agglomerate formation at one interface by considering hydrophobic and electrostatic interactions. Liu et al. investigated the stability of nano-SiO2 in the presence of a cationic surfactant30 and demonstrated that different mechanisms of ENP agglomeration (charge neutralization, depletion flocculation and hydrophobic effects) were involved in the ENP destabilization. Coagulation–flotation processes of inorganic ENPs in the presence of cationic surfactants were also investigated to optimize the removal of ENPs through particle agglomeration for the treatment of wastewaters from industries producing silica ENPs.31–33 Other studies dealing with ENP–surfactant interactions investigated the structures formed during the complexation processes between bare (or polymer coated) ENPs and surfactants by using Fourier transform infrared spectroscopy,34 small-angle neutron scattering35–37 and solvent relaxation nuclear magnetic resonance.38 In these studies, the authors observed that the presence of surfactants promoted the desorption of the polymer coating due to the conformational change of the polymer layer to a more diffuse state.
Since surfactants are largely used in the field of nanotechnology they are likely to be one of the chemicals that will coexist with ENPs in the environment. Indeed ENPs have the possibility to contaminate water treatment plants, in which surfactants are present, as well as aquatic systems through direct, indirect, accidental or deliberate ENP release.39–41 Current knowledge suggests that ENPs entering aquatic systems will exhibit transport and fate behavior that will be highly dependent both on their released form and on the physicochemical characteristics of the receiving water.42–46 There is thus a great need to evaluate the effects of water chemistry, changing conditions, and stability of the complexes they form with surfactants under environmental conditions.
In this study, we are investigating the properties and stability of TiO2 ENPs in the presence of sodium dodecyl sulfate (SDS) which is a common anionic surfactant with low production cost.47 We first focus on the effect of the SDS concentration (and how SDS is added into the ENP dispersions, i.e. punctual versus successive additions) on TiO2 stability in a pH domain where electrostatic interactions between the two compounds are favored. Formation of electrostatic complexes based on electrostatic attractions is of great importance for the synthesis of stable ENP dispersions via surfactant surface coating. Then, the TiO2 stability in the presence of SDS, by considering surface charge and size changes, is evaluated upon pH variations and in the presence of divalent cations to investigate the effects of water hardness. This enables the release and behavior of surfactant coated, stable, ENP dispersions in natural waters to be mimicked, which is of high importance for the risk assessment associated with ENPs entering aquatic systems. The novelty of this study is to consider here different scenarios (SDS concentration variations, pH changes, the presence of divalent cations) and systematic measurements of surface charges and z-average sizes to propose a detailed mechanistic approach and predict the TiO2 behavior from pristine solutions to aquatic systems in the presence (or not) of surfactants. The importance of electrostatic and hydrophobic interactions, SDS desorption and specific adsorption of divalent cations is here discussed by considering stabilization, agglomeration and disagglomeration processes.
2. Materials and methods
2.1. Materials
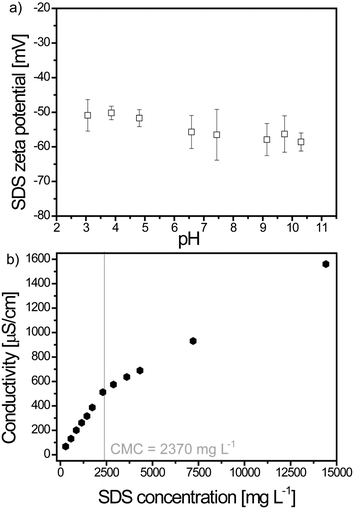
1 g L−1 dispersions were prepared by dispersing anatase TiO2 ENP powder (Nanostructured & Amorphous Material Inc, Houston, TX, USA) in ultrapure Milli Q water (Millipore, Zoug, ZG, Switzerland, with R > 18 MΩ cm, T.O.C. < 2 ppb). The pristine diameter and specific surface area were, as given by the manufacturer, equal to 15 nm (TEM) and 240 m2 g−1 (BET). The dispersions were sonicated with an ultrasonic probe (CV18, Sonics Vibra cell, Blanc Labo S.A., Switzerland) delivering a power of 50 W for 15 min using a pulsed 80% mode.48 Sodium dodecyl sulfate (AppliChem, purity >99%, Darmstadt, Germany) solutions were prepared by dissolving the surfactant in ultrapure Milli Q water and used without further purification. To adjust the dispersion pH, sodium hydroxide and hydrochloric acid (1 M NaOH or HCl, Titrisol®, Merck, Zoug, ZG, Switzerland) were used after dilution. Calcium chloride (CaCl2, purum, Fluka, Buchs, SG, Switzerland) and magnesium chloride (MgCl2, puriss, Sigma Aldrich, Buchs, SG, Switzerland) were used to adjust the electrolyte concentration to the desired values. Experiments were performed in 25 × 90 mm polypropylene tubes (Milian, Vernier, GE, Switzerland) with one 8 × 10 mm crosshead magnetic stirrer (VWR, Nyon, VD, Switzerland) and the agitation speed was set to 500 rpm.2.2. Zeta potential and size distribution measurements
Determination of z-average hydrodynamic diameters and zeta (ζ) potential values was achieved by dynamic light scattering and laser doppler velocimetry using a Zetasizer Nano ZS instrument (Malvern Instruments, Worcestershire, UK). The Smoluchowski approximation model49,50 was applied for ζ potential determination. Analysis was conducted at 25 °C and by considering in most cases 50 mg L−1 TiO2 dispersions. Such a concentration was selected to provide an optimum light scattering signal. All polydispersity indexes were found below 0.6. All measurements were made 30 min after pH changes or SDS addition.2.3. Experiments under pH changing conditions and in the presence of divalent electrolytes
The influence of pH changes (from acidic to circumneutral pH, representative of natural aquatic systems) in the presence of divalent cations on the stability of TiO2–SDS complexes was investigated by first forming stable complexes at low pH. Then they were introduced into a synthetic water containing divalent cations so as to take into account water hardness. The Ca2+ and Mg2+ concentrations were, after addition of TiO2–SDS complexes, equal to 45 mg L−1 and 5 mg L−1, the pH to 8.2 and the TiO2 concentration to 25 mg L−1. The total hardness and pH of investigation were adjusted to the values generally found in the Geneva Lake (France, Switzerland).2.4. Procedure of SDS addition: punctual addition and successive addition
The effect of the SDS surfactant on the stability of TiO2 ENPs is investigated for two different scenarios of SDS addition. For “punctual” addition, the total amount of SDS is injected into the TiO2 dispersion in a unique manner so that the final SDS concentration is directly obtained after the addition. For “successive” addition, the ENP dispersion is titrated with the SDS solution to finally reach, after a certain number of injections, the final desired SDS concentration. The time between each successive addition was set to 30 min which corresponds here to the time needed to obtain a plateau in terms of zeta potential and hydrodynamic diameter values.3. Results and discussion
3.1. Influence of SDS concentration on TiO2 ENP properties: Punctual versus successive addition of SDS
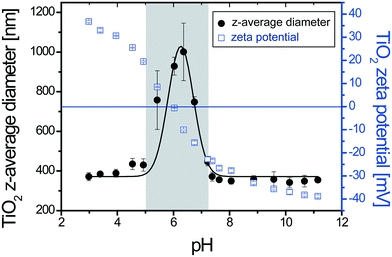
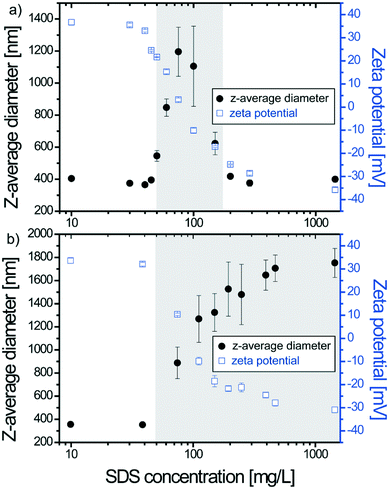
The influence of the SDS concentration on the stability of 50 mg L−1 TiO2 dispersions is first investigated at pH 3.1 under conditions where the ENP surfaces are, prior SDS addition, positively charged (Fig. 1) with a ζ potential value equal to +36 mV. All SDS concentrations used during the experiments are below the CMC and two mixing modes are considered (punctual versus successive SDS addition).3.2. Behavior of SDS–TiO2 complexes upon pH variation
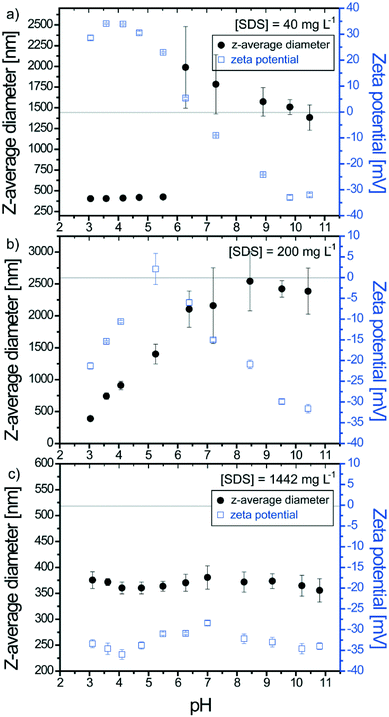
The effect of pH variation (from acid to basic pH with 30 min between successive pH adjustments) on stable SDS–TiO2 electrostatic complexes formed at pH 3.1 is now investigated at a constant TiO2 concentration (50 mg L−1) and by considering three SDS concentrations. The first concentration is equal to 40 mg L−1 and results in the formation of positively charged complexes at pH 3.1. The second one is equal to 200 mg L−1 and results in the formation of negatively charged complexes. The third concentration corresponds to 1442 mg L−1 and corresponds to the formation of negatively charged complexes with a large excess of SDS.As shown in Fig. 5a when partial coating of TiO2 is obtained at 40 mg L−1 the increase of pH to 5.5 does not affect the complex stability (constant z-average diameters) even if the deprotonation of TiO2 hydroxyl surface sites results in a decrease of the ζ potential from +30 mV to +25 mV. Further pH increase rapidly promotes surface charge neutralization then formation of agglomerates (sizes >1 μm). Agglomerate formation is expected to be due here mainly to TiO2 surface charge neutralization owing to the limited number of adsorbed SDS molecules. It is noteworthy that the IEP is found equal to pH 6.5 ± 0.1 which is higher than the PZC value obtained in the absence of SDS (pHPZC = 6.1). Such a small but significant difference is attributed to the difficulties in deprotoning the TiO2 positive surface due to the presence of the negatively charged SDS molecules which changes the acid–base properties of the TiO2 surface groups. By increasing further the pH (pH ≥ 10) a continuous decrease of the mean z-average diameters is observed. The sizes of such agglomerates are greater than those of agglomerates formed at pH 3.1 or agglomerates formed at pH 10 by punctual addition (Fig. 1). This is due to the successive pH adjustment and the fact that once formed, large agglomerates only undergo partial disagglomeration. High pH values result in charge inversion to negative values which are close to the values obtained in the absence of surfactants hence indicating desorption of the negatively charged surfactants from the negatively charged ENP surfaces. Under such conditions of low SDS concentration, the TiO2–SDS complex properties and its pH charge dependency are mainly controlled by the TiO2 surface properties.
ζ potential values and z-average diameters of TiO2–SDS complexes formed at 200 mg L−1 SDS concentration are presented in Fig. 5b. At pH 3.1 the complexes are negatively charged and the pH increase rapidly results in a decrease of the ζ potential and concomitant formation of agglomerates. Such a behavior is related here to SDS molecule desorption when positive surface sites are deprotonated as well as the presence of both positive and negative sites on the ENP surface when the ζ potential is equal to zero. The IEP is obtained here at pH 5.2 and corresponds to a state where the number of hydroxyl positive surface sites is still important but exactly counterbalanced by the adsorbed SDS molecules. Further pH increase, from pH 6 to 10, induces TiO2 surface site deprotonation and a continuous decrease to more negative values of the ζ potential. The size of the agglomerates increases continuously with pH increase from 3 to 8.5 then stabilization is observed. No significant disagglomeration is observed.
Size and surface charge evolution as a function of pH for TiO2–SDS complexes obtained with an important excess of surfactants (SDS concentration equal to 1442 mg L−1) is represented in Fig. 5c. In the full pH domain, the complexes are found stable with constant z-average diameters and ζ potential values of about 370 nm and −34 mV, respectively. Data indicate here that the SDS–TiO2 properties are essentially controlled by the presence of the surfactants and that the TiO2 surface deprotonation has little effect on the complex total charge which is held constant by the desorption of SDS molecules. An excess of SDS molecules in comparison with the experiment previously discussed in the presence of 200 mg L−1 prevents a decrease of the ζ potential value upon pH variation and therefore formation of large agglomerates.
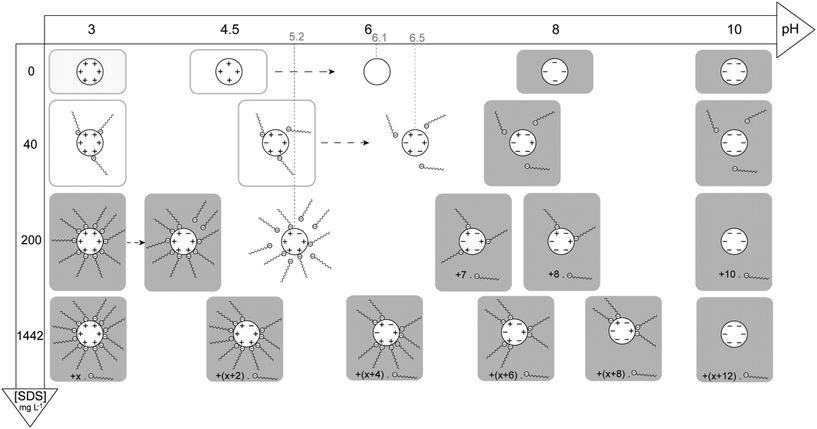
The TiO2–SDS complexes, relative charges, number of SDS molecules and stability diagram with pH have been summarized in Fig. 6. The number of charges on TiO2 ENPs and number of SDS molecules are only qualitative to take into account the total surface charge of the ENPs with respect to the experimental ζ potential values. This stability diagram denotes the subtle interplay between the TiO2 ENP surface charge, pH, SDS concentration and impacts on SDS adsorption, SDS desorption, agglomeration as well as disagglomeration processes.
3.3. Stability of TiO2–SDS complexes in the presence of divalent cations and at environmental pH
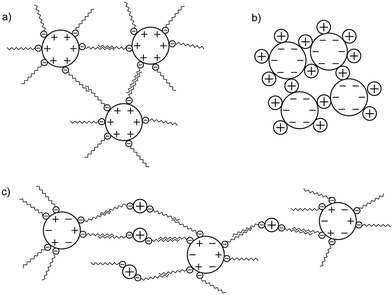
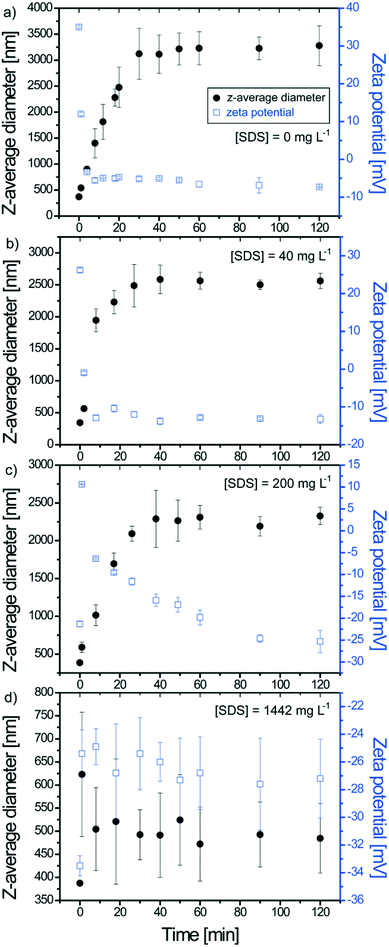
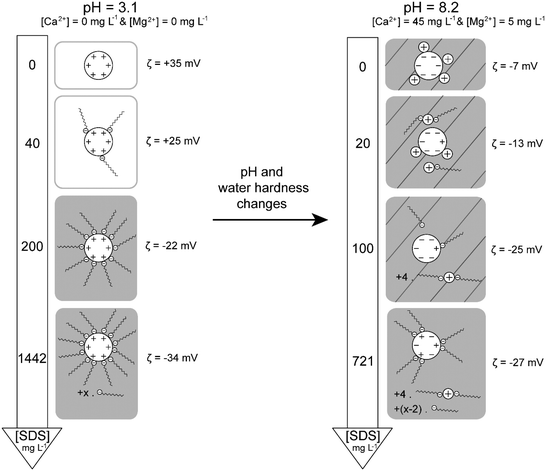
The stability of TiO2–SDS complexes is investigated by first forming electrostatic and stable complexes at pH 3.1 and then by introducing them in synthetic water at pH 8.2, with [Ca2+] = 45 mg L−1 and [Mg2+] = 5 mg L−1 so as to take into account water hardness. ζ potential value and z-average diameter evolutions as a function of time for different initial SDS concentrations are represented in Fig. 7. In the absence of SDS, when positively charged TiO2 ENPs are introduced in the synthetic water (Fig. 7a), TiO2 charge inversion is observed and the ζ potential rapidly changes from a positive value to −7 mV. It should be noted here that this final ζ potential value is lower than that observed in the absence of divalent cations (ζ potential equal to −30 mV at pH 8.2 as shown in Fig. 1). Such a difference is explained by the specific adsorption of divalent cations on the ENP surface in addition to charge screening. Owing to the ζ potential value, large agglomerates are then rapidly formed and, after 30 min, a steady state is obtained. The presence of divalent cations is also found to promote the formation of large agglomerates (diameters up to 3 μm) with the help of cation bridging as represented in Fig. 4b and in comparison with agglomerate diameters obtained in the absence of divalent electrolytes (around 1 μm at pHPZC after 30 min).For a 40 mg L−1 SDS concentration (Fig. 7b) similar trends are observed. However, the final ζ potential values are found more negative in the presence of SDS (−13 versus −7 mV). Under such conditions Ca2+ and Mg2+ ions are assumed to form complexes with the SDS molecules and, as a result, less divalent cations are available for specific adsorption on TiO2 surfaces.
For a 200 mg L−1 SDS concentration (Fig. 7c) the ζ potential stabilization takes a longer time (90 min instead of 30 min in the previous experiment) due to TiO2 surface charge modification and equilibrium time between SDS desorption and SDS–cation complex formation. Indeed, we start with a negative surface potential which rapidly becomes positive then decreases again to negative values. The ζ potential plateau obtained here is higher than that obtained in the absence of divalent cations for an identical pH (−25 versus −20 mV in Fig. 5b) as SDS–cation complex formation reduces the SDS amount adsorbed. Finally, for the highest SDS concentration investigated (1442 mg L−1, Fig. 7d) a significant increase of the z-average diameter is observed, if comparison is made in the absence of cations, due to cation bridging between TiO2–SDS complexes as represented in Fig. 4c. The ζ potential reaches a constant value equal to −27 mV which is lower than that observed in the absence of Ca2+ and Mg2+ (−34 mV at pH 8.2) due to SDS–cation complex formation. Less SDS molecules are then available for adsorption on the TiO2 surface. Fig. 8 summarizes the influence of the pH change and the presence of divalent cations on the resulting stability and structure of TiO2–SDS complexes. Agglomeration conditions are presented in this figure by hatched squares.
4. Conclusion
In this study, using a mechanistic approach, we demonstrate that surfactant molecules can significantly influence ENP stability through several processes. The surfactant concentration, mixing procedure (punctual or successive addition), pH as well as the presence of divalent cations are also found to be of high importance.Stabilization/destabilization processes are shown here to be governed by complex mechanisms such as electrostatic repulsions, hydrophobic interactions between SDS chains, specific adsorption of divalent cations, adsorption/desorption of surfactant molecules, and cation bridging. It should be also noted that for a given SDS concentration, electrostatic TiO2–SDS complexes can undergo important transformation with pH. We believe that this study is of high value for i) the design of ENP dispersion protocols to improve nanoparticle stability with the use of surfactant molecules, ii) a better understanding of ENP commercial dispersions stabilized by surfactants in contact with environmental matrices, iii) the understanding of the impact of water hardness on the SDS–TiO2 nanoparticle interactions in particular by considering the competition between the divalent cations, surfactant molecules and TiO2 nanoparticles, and iv) for risk assessment evaluation of ENPs entering aquatic systems where both changes in pH and water hardness are expected.
Acknowledgements
The authors are grateful to the financial support received from the Swiss National Foundation (project numbers 200020_152847 and 200021_135240). This work was partially funded via the European Commission's 7th Framework project “NanoMILE” (contract no. NMP4-LA-2013-310451).References
- M. Auffan, J. Rose, J.-Y. Bottero, G. V. Lowry, J.-P. Jolivet and M. R. Wiesner, Nat. Nanotechnol., 2009, 4, 634–641 CrossRef CAS PubMed.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- A.-H. Lu, E. L. Salabas and F. Schueth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- X. L. Luo, A. Morrin, A. J. Killard and M. R. Smyth, Electroanalysis, 2006, 18, 319–326 CrossRef CAS.
- C. O. Hendren, X. Mesnard, J. Droege and M. R. Wiesner, Environ. Sci. Technol., 2011, 45, 2562–2569 CrossRef CAS PubMed.
- K. Schmid and M. Riediker, Environ. Sci. Technol., 2008, 42, 2253–2260 CrossRef CAS PubMed.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, J. Nanopart. Res., 2012, 14, 1109 CrossRef.
- M. Gratzel, J. Photochem. Photobiol., A, 2004, 168, 235 CrossRef.
- A. Weir, P. Westerhoff, L. Fabricius, K. Hristovski and N. von Goetz, Environ. Sci. Technol., 2012, 46, 2242–2250 CrossRef CAS PubMed.
- B. Mahltig, H. Bottcher, K. Rauch, U. Dieckmann, R. Nitsche and T. Fritz, Thin Solid Films, 2005, 485, 108–114 CrossRef CAS.
- P. D. Cozzoli, R. Comparelli, E. Fanizza, M. L. Curri and A. Agostiano, Mater. Sci. Eng., C, 2003, 23, 707–713 CrossRef.
- X. Jimin, J. Deli, C. Min, L. Di, Z. Jianjun, L. Xiaomeng and Y. Changhao, Colloids Surf., A, 2010, 372, 107–114 CrossRef.
- R. A. French, A. R. Jacobson, B. Kim, S. L. Isley, R. L. Penn and P. C. Baveye, Environ. Sci. Technol., 2009, 43, 1354–1359 CrossRef CAS PubMed.
- R. F. Domingos, C. Peyrot and K. J. Wilkinson, Environ. Chem., 2010, 7, 61–66 CrossRef CAS.
- B. Mukherjee and J. W. Weaver, Environ. Sci. Technol., 2010, 44, 3332–3338 CrossRef CAS PubMed.
- J. A. Gallego-Urrea, J. Perez Holmberg and M. Hassellov, Environ. Sci.: Nano, 2014, 1, 181–189 RSC.
- M. Baalousha, A. Manciulea, S. Cumberland, K. Kendall and J. R. Lead, Environ. Toxicol. Chem., 2008, 27, 1875–1882 CrossRef CAS PubMed.
- M. Erhayem and M. Sohn, Sci. Total Environ., 2014, 468–469, 249–257 CrossRef CAS PubMed.
- O. Spalla, Curr. Opin. Colloid Interface Sci., 2002, 7, 179–185 CrossRef CAS.
- E. Bae, H.-J. Park, J. Park, J. Yoon, Y. Kim, K. Choi and J. Yi, Bull. Korean Chem. Soc., 2011, 32, 613–619 CrossRef CAS.
- M. D. Clark, S. Subramanian and R. Krishnamoorti, J. Colloid Interface Sci., 2011, 354, 144–151 CrossRef CAS PubMed.
- K. Holmberg, J. Colloid Interface Sci., 2004, 274, 355–364 CrossRef CAS PubMed.
- L. R. Parent, D. B. Robinson, T. J. Woehl, W. D. Ristenpart, J. E. Evans, N. D. Browning and I. Arslan, ACS Nano, 2012, 6, 3589–3596 CrossRef CAS PubMed.
- G. G. Ying, Environ. Int., 2006, 32, 417–431 CrossRef CAS PubMed.
- http://www.cefic.org/About-us/How-Cefic-is-organised/Fine-Speciality-and-Consumer-Chemicals/Comite-Europeen-des-Agents-de-Surface-CESIO-/- (Accessed July 13, 2016).
- E. Samper, M. Rodriguez, M. A. De la Rubia and D. Prats, Sep. Purif. Technol., 2009, 65, 337–342 CrossRef CAS.
- X. Li, G.-M. Zeng, J.-H. Huang, C. Zhang, Y.-Y. Fang, Y.-H. Qu, F. Luo, D. Lin and H.-L. Liu, J. Membr. Sci., 2009, 337, 92–97 CrossRef CAS.
- T. Imae, K. Muto and S. Ikeda, Colloid Polym. Sci., 1991, 269, 43–48 CAS.
- C. Arnold, S. Ulrich, S. Stoll, P. Marie and Y. Holl, J. Colloid Interface Sci., 2011, 353, 188–195 CrossRef CAS PubMed.
- Y. Liu, M. Tourbin, S. Lachaize and P. Guiraud, Chemosphere, 2013, 92, 681–687 CrossRef CAS PubMed.
- Y. Liu, M. Tourbin, S. Lachaize and P. Guiraud, Ind. Eng. Chem. Res., 2012, 51, 1853–1863 CrossRef CAS.
- C. Y. Lien and J. C. Liu, J. Environ. Eng., 2006, 132, 51–57 CrossRef CAS.
- C. Y. Hu, S. L. Lo, C. M. Li and W. H. Kuan, J. Hazard. Mater., 2005, 120, 15–20 CrossRef CAS PubMed.
- H. Li and C. P. Tripp, Langmuir, 2004, 20, 10526–10533 CrossRef CAS PubMed.
- S. Mehan, V. K. Aswal and J. Kohlbrecher, Langmuir, 2014, 30, 9941–9950 CrossRef CAS PubMed.
- S. Mehan, A. J. Chinchalikar, S. Kumar, V. K. Aswal and R. Schweins, Langmuir, 2013, 29, 11290–11299 CrossRef CAS PubMed.
- K. P. Sharma, V. K. Aswal and G. Kumaraswamy, J. Phys. Chem. B, 2010, 114, 10986–10994 CrossRef CAS PubMed.
- B. Cattoz, T. Cosgrove, M. Crossman and S. W. Prescott, Langmuir, 2012, 28, 2485–2492 CrossRef CAS PubMed.
- N. Sani-Kast, M. Scheringer, D. Slomberg, J. Labille, A. Praetorius, P. Ollivier and K. Hungerbuhler, Sci. Total Environ., 2015, 535, 150–159 CrossRef CAS PubMed.
- G. E. Batley, J. K. Kirby and M. J. McLaughlin, Acc. Chem. Res., 2013, 46, 854–862 CrossRef CAS PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- S. M. Louie, R. D. Tilton and G. V. Lowry, Environ. Sci.: Nano, 2016, 3, 283–310 RSC.
- G. Cornelis, Environ. Sci.: Nano, 2015, 2, 19–26 RSC.
- E. Goldberg, M. Scheringer, T. D. Bucheli and K. Hungerbuhler, Environ. Sci.: Nano, 2015, 2, 352–360 RSC.
- B. Collin, M. Auffan, A. C. Johnson, I. Kaur, A. A. Keller, A. Lazareva, J. R. Lead, X. Ma, R. C. Merrifield, C. Svendsen, J. C. White and J. M. Unrine, Environ. Sci.: Nano, 2014, 1, 533–548 RSC.
- L. R. Pokhrel, B. Dubey and P. R. Scheuerman, Environ. Sci.: Nano, 2014, 1, 45–54 RSC.
- A. Biswal, B. C. Tripathy, T. Subbaiah, D. Meyrick and M. Minakshi, J. Electrochem. Soc., 2015, 162, 30–38 CrossRef.
- J. S. Taurozzi, V. A. Hackley and M. R. Wiesner, National Institute of Standards and Technology Special Publication, 2012, pp. 1200–1203 Search PubMed.
- M. Baalousha, Sci. Total Environ., 2009, 407, 2093–2101 CrossRef CAS PubMed.
- H. Ohshima, Adv. Colloid Interface Sci., 1995, 62, 189–235 CrossRef CAS.
- G. A. Parks, Chem. Rev., 1965, 65, 177–198 CrossRef CAS.
- A. Praetorius, J. Labille, M. Scheringer, A. Thill, K. Hungerbuehler and J.-Y. Bottero, Environ. Sci. Technol., 2014, 48, 10690–10698 CrossRef CAS PubMed.
- B. Faure, G. Salazar-Alvarez, A. Ahniyaz, I. Villaluenga, G. Berriozabal, Y. R. De Miguel and L. Bergstrom, Sci. Technol. Adv. Mater., 2013, 14, 023001 CrossRef PubMed.
- A. M. Khan and S. S. Shah, J. Chem. Soc. Pak., 2008, 30, 186–191 CAS.
- A. Cifuentes, J. L. Bernal and J. C. DiezMasa, Anal. Chem., 1997, 69, 4271–4274 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |