Multi-day diurnal measurements of Ti-containing nanoparticle and organic sunscreen chemical release during recreational use of a natural surface water†
R. B.
Reed
a,
D. P.
Martin
b,
A. J.
Bednar
b,
M. D.
Montaño
c,
P.
Westerhoff
d and
J. F.
Ranville
*a
aDepartment of Chemistry and Geochemistry, Colorado School of Mines, Golden, CO 80401, USA. E-mail: jranvill@mines.edu
bU. S. Army Engineer Research and Development Center, Environmental Laboratory, Vicksburg, MS 39180, USA
cCenter for Environmental Implications of Nanotechnology, Department of Civil and Environmental Engineering, Duke University, Durham, NC 27708, USA
dSchool of Sustainable Engineering and the Built Environment, Arizona State University, Tempe, AZ 85287, USA
First published on 14th November 2016
Abstract
Clear Creek in Golden, Colorado sees a large number of recreational users during summer, which is expected to result in release of sunscreen chemicals to the water. In this study, water samples were collected hourly for 72 hours over a busy holiday weekend, and were analyzed for the organic chemical – based (oxybenzone) and inorganic colloidal (titanium dioxide) active sunscreen constituents. An increase in oxybenzone concentration was observed daily during each day's peak recreational use, approximately 12:00 to 19:00 h. This corresponded with an increase in titanium concentration. Metals naturally co-occurring with titanium such as aluminum and iron also showed an increase of these elements during bathing periods as well, suggesting the titanium increase may also be partially the result of sediment resuspension, consistent with the shallow water depth. The ratio of titanium to both aluminum and iron increases relative to the background elemental ratios during peak recreational use. Estimates of titanium mass loading suggested that sunscreen use only could not explain the observed Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al and Ti
Al and Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratios and that resuspended sediments likely have an elevated titanium metal ratio compared to natural suspended sediments. Single particle ICP-MS (spICP-MS), used to analyze water samples for Ti-containing particles, did not show diurnal trends in total particle number. Overall, this is the first consecutive-multi-day monitoring study for compounds released from sunscreen to a natural water system, and it highlights the challenges in dealing with detection of NPs above a natural background.
Fe ratios and that resuspended sediments likely have an elevated titanium metal ratio compared to natural suspended sediments. Single particle ICP-MS (spICP-MS), used to analyze water samples for Ti-containing particles, did not show diurnal trends in total particle number. Overall, this is the first consecutive-multi-day monitoring study for compounds released from sunscreen to a natural water system, and it highlights the challenges in dealing with detection of NPs above a natural background.
Environmental significanceIn order to better understand the behavior of nanomaterials in natural systems used by humans, we studied release of nano-TiO2 during summer recreational activity in a popular Colorado waterway. Hourly measurements were made over a three-day weekend in summer, both upstream and downstream of the recreational area. For confirmation that sunscreen was being released by bathers, oxybenzone, an organic chemical used in sunscreen, was measured in surface water samples as well, and showed increase during recreational activities concurrently with Ti. To our knowledge, this is the first study to measure both metal and organic chemical release from sunscreen in natural surface water. This study provides a framework for environmental release analysis from other nano-enabled consumer products in addition to sunscreen. |
Introduction
With the increased use of engineered nanoparticles (NPs) in consumer products, concern about their potential for release to the environment has risen. Most studies that model release of NPs to the environment have suggested that very low (ca. ng L−1) concentrations1–3 are expected, requiring highly sensitive metrology for accurate detection, quantification, and characterization of these materials in complex environmental matrices. In addition to detecting low concentrations of engineered NPs, differentiating them from natural NPs is also a significant challenge.Many release scenarios for NPs involve consumer use in the home such as washing of NP-containing textiles,4–8 release from industry such as chemical mechanical planarization NPs (e.g. Al2O3, CeO2, SiO2),9 and subsequent release from wastewater treatment plants (WWTP) to the environment.10 For NPs in products used in natural waters, such as TiO2 NPs in sunscreen, the release is more direct. Gondikas et al.11 measured variations in Ti over the course of a year including summer bathing in Vienna, Austria, and suggested that an increase in the Ti/Al ratio indicated detection of engineered NPs released by bathers. Holbrook et al. measured Ti concentration in a swimming pool in the 20–60 μg L−1 range, with the majority of Ti being dissolved (below 1 kDa in size).12 The work reported here seeks to add to this limited existing data set on engineered TiO2 in natural waters.
A variety of analytical techniques are applicable to measuring NPs released to the environment. Electron microscopy has been used,11 however this is extremely time-consuming and non-quantitative for determining NP mass or number concentrations. In addition, the particles measured may not be truly representative of the environmental state of the NPs due to possible sample preparation artifacts. Bulk measurements by inductively coupled plasma – atomic emission spectroscopy (ICP-AES) or – mass spectrometry (ICP-MS) are useful for measuring the change in total metal concentrations possibly due to NP inputs over time.11 Single particle ICP-MS (spICP-MS) is promising for its ability to detect and size a variety of individual particles13–17 and to quantify particle number concentrations. However, this technique does not directly provide differentiation between engineered and natural NPs. Work on multi-element detection in each single particle by spICP-MS18,19 is promising for addressing this challenge, but further research remains to be done in this area.
Modeling studies on the predicted concentrations of NPs in the environment provide average concentrations over a broad region's environmental compartments such as soil, air, water, etc.2,20 However, releases greater than these predicted averages are likely to occur in localized regions. In the case of TiO2 NPs used in sunscreen, this is likely to occur in heavily used bathing areas.11 Clear Creek, a natural waterway running through Golden, Colorado, sees a large increase in recreational use during summer. With summer temperatures often reaching 38 °C, people use Clear Creek for swimming, wading, tubing, and kayaking. Frequent and intense sunlight, coupled with its high elevation (1700 m above sea level) leads to increased UV exposure, making sunscreen use in Colorado important for reducing sunburn and skin cancer risk during outdoor recreational activities. Labor day weekend, a three-day weekend in the U.S. that occurs in early September, is typically one of the busiest recreational periods on Clear Creek, making this an ideal time to monitor for TiO2 NP release from sunscreen. People recreate in the water by swimming and floating in inflatable devices (e.g., tubes).
In comparison to the lack of data on TiO2 occurrence in the environment, significantly more information is available on the occurrence and fate of organic chemical sunblock agents. These are often functionalized phenols such as oxybenzone, which is used as an active ingredient in sunscreen.21
The goal of this study was twofold: to use multiple analytical approaches to attempt detection of engineered TiO2 NPs in surface waters, and to assess the challenges associated with quantifying these NPs above background in a natural surface water. An organic chemical sunscreen agent (oxybenzone) was analyzed by HPLC to contrast against our current analytical detection of nanomaterials in water (i.e., TiO2 sunscreen agents). Water samples were collected hourly from Clear Creek over the three - day weekend. In addition, metals (iron and aluminum) that naturally co-occur with titanium in suspended mineral particles were measured. Single particle ICP-MS (spICP-MS) analysis was performed on water samples to quantify the number of Ti-containing particles. Overall, this study highlights the challenges in detecting and quantifying engineered NPs in the presence of natural particles, in this case natural Ti-containing particles.
Methods and materials
Sample collection
Surface water samples were collected from Clear Creek hourly over a 72 hour period from September 5–7, 2015 using automated ISCO samplers (Teledyne Isco, Lincoln, NE). The two samplers were placed approximately 1 km apart, both upstream and downstream of the majority of recreational activity in the creek (images shown in Fig. S1–S3†). Water samples were split into three containers for storage before analysis: pre-cleaned 250 mL high density polyethylene (HDPE) bottles containing 1 mL ultrapure nitric acid (OmniTrace grade) were used for total metals analysis, 500 mL amber glass bottles were used for oxybenzone analysis (samples preserved by refrigeration only), and water for spICP-MS analysis was placed in polypropylene centrifuge tubes (Fischer Scientific, Waltham, MA). Samples were stored under refrigeration at 4 °C.Reagents and supplies
All chemicals used were of reagent grade or higher purity and used without further purification; the deionized water used had a resistivity of 18.2 MΩ cm. Nitric acid (OmniTrace grade) used for metals analysis was purchased from Fisher Scientific. Methanol and acetonitrile (HPLC grade) used for organic sunscreen analysis were purchased from JT Baker (Center Valley, PA). Solid phase extraction (SPE) cartridges (Sep-Pak Porapak RDX polydivinylbenzene-vinylpyrrolidone) were purchased from Waters Corporation (Milford, MA).Trace organics analysis
Concentrations of oxybenzone, an organic sunscreen compound, were determined in water samples using high performance liquid chromatography with UV absorbance detection at 315 and 365 nm, after SPE pre-concentration as described by Peck et al.22 Briefly, 400 mL of non-preserved water was passed through an SPE cartridge to concentrate organic compounds such as oxybenzone, which were subsequently eluted in 8 mL of acetonitrile with 0.1% trifluoroacetic acid. The extracts were then concentrated via evaporation to 4 mL resulting in a 100× final concentration factor. The extracts were placed in autosampler vials and diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with deionized water (calibration standards were treated identically, resulting in no effect upon the 100× concentration factor). An Agilent (Santa Clara, CA) 1200 HPLC equipped with a Phenomonex Synergi (Torrance, CA) 4u Hydro-RP 80A HPLC column was used for analysis. Fifty μL of the sample was injected, with elution by a 90
1 with deionized water (calibration standards were treated identically, resulting in no effect upon the 100× concentration factor). An Agilent (Santa Clara, CA) 1200 HPLC equipped with a Phenomonex Synergi (Torrance, CA) 4u Hydro-RP 80A HPLC column was used for analysis. Fifty μL of the sample was injected, with elution by a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 methanol
10 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water isocratic mobile phase. All standards for sunscreen determination by HPLC-UV were purchased from U.S. Pharmacopeia (Rockville, MD).
water isocratic mobile phase. All standards for sunscreen determination by HPLC-UV were purchased from U.S. Pharmacopeia (Rockville, MD).
Total metals analysis
Metal concentrations were determined on nonfiltered water samples acidified with nitric acid using ICP-AES or ICP-MS, as appropriate, for the concentration ranges observed for major and trace elements following modifications of methods 6010C and 6020A23 using a Perkin Elmer (Wellesley, MA) Optima 8300DV ICP-AES or NexION 300D ICP-MS. All analytical standards for metals determination by ICP-AES and ICP-MS were NIST-traceable, and purchased from SPEX CertiPrep (Metuchen, NJ) and CPI International (Santa Rosa, CA).Calibration and check standards were diluted in 1% trace-metal grade nitric acid to appropriate calibration ranges (1–100 μg L−1 for ICP-MS and 0.1 to 10 mg L−1 for ICP-AES). Scandium, germanium, yttrium, rhodium, terbium, and holmium were added on-line with a mixing-T prior to introduction into the nebulizer for use as internal standards. Variations in internal standard intensity were less than 15% over the course of an analytical batch. Continuing calibration and independent second source verification standards were analyzed periodically in each analytical batch with recovery for all analytes within 10% of the certified value; additionally, a commercially available reference solution from Environmental Resource Associates (Golden, CO; Lot number P136-500) was analyzed in each analytical batch with analyte recoveries within 15% of the certified value. Duplicate and matrix spike samples were included in each analytical batch, with duplicate precision demonstrated by relative percent differences of <20% and matrix spike recoveries within 80–120% of the nominal spike concentration for all analytes.
Single particle ICP-MS analysis
Creek water samples selected from both peak and off-peak recreation times were analyzed by spICP-MS using a Perkin Elmer (Wellesley, MA) NexION 300D ICP-MS. A 3 millisecond dwell time was used for time-resolved data collection. The instrument transport efficiency was measured (∼5%) with each analysis batch using a British Biocell International 60 nm Au NP. Dissolved standards used were SPEX Certiprep (Metuchen, NJ) ICP-MS grade standards. The isotope used was 49Ti (5.42% natural abundance), in order to reduce isobaric interferences, especially 48Ca and 32S16O, which prevent the use of 48Ti (73.7% natural abundance) on the quadrupole-based instruments.A Thermo (Waltham, MA) Element II High Resolution ICP-MS (HR-ICP-MS) was used in single particle mode to evaluate the size detection limit for TiO2 for a high resolution ICP-MS compared to a quadrupole ICP-MS, also using 3 millisecond dwell times. The instrument transport efficiency was measured with each analysis batch using a British Biocell International 60 nm Au NP. Dissolved standards used were PlasmaCAL (SCP Science, Baie-d'Urfé, Quebec) ICP-MS grade standards, diluted in the range of 5 to 5000 ng L−1 in 1% ultrapure nitric acid.
Results and discussion
Oxybenzone and Ti concentrations increase during creek recreation
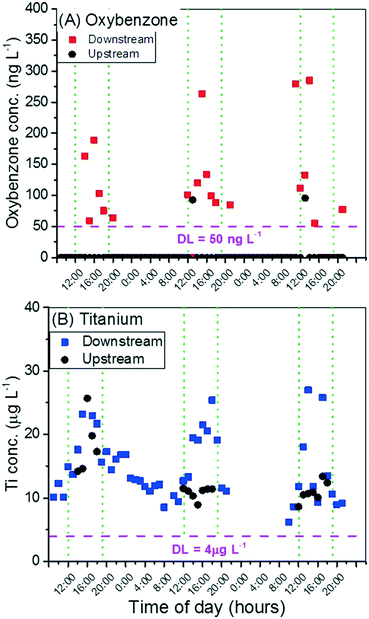
Peak recreational activity occurred in the afternoon each of the three days over the weekend, typically over the period 12:00–18:00 as qualitatively observed by researchers collecting samples. Photos (Fig. S1–S3†) were taken from a consistent location near the downstream ISCO sampler during the daylight hours (9:00 to 19:00). Although non-quantitative, this helps identify the heavy recreational use periods.As seen in Fig. 1A, the downstream oxybenzone concentration increases during the afternoons and evenings (12:00 to 19:00 most days), an indicator of sunscreen use by swimmers and bathers. During the night and early morning, oxybenzone levels are below the method detection limit of 50 ng L−1. Except for two afternoon samples, the concentration of oxybenzone upstream is consistently below detection limit as well. There is a small amount of recreational use above the upstream sampling site, mostly by people floating down the creek on inner tubes, and this usage could have resulted in a measurable contribution of oxybenzone for the upstream samples.
The concentration of Ti increases above background concurrently each day with the increase in oxybenzone concentration (Fig. 1B). This initially suggests detection of engineered TiO2 released from sunscreens during recreational use, but the magnitude of the [Ti] increase is much greater, ∼10 μg L−1 above the morning and night background each afternoon as compared with the oxybenzone increase of 50 to 300 ng L−1. It is possible, but unlikely, that many more people were using TiO2-containing sunscreens, or that the TiO2 concentration in sunscreens was much higher than those containing oxybenzone (discussed later). It is also possible that the recreational activity in the creek is causing bioturbation of sediment, which contributes to the concentration increase of Ti in the water column, associated with natural sediment. Titanium is the 9th most frequently occurring element in the earth's crust. The photos taken (Fig. S3†) also illustrate that in many sections of the creek, people are able to stand on the creek bed, possibly disturbing the bed sediment. Most likely the observed increase in Ti results as a combination of engineered TiO2 and natural Ti-containing particles.
In addition to the downstream [Ti] increase with recreational activity, an apparent increase in [Ti] upstream was observed on the first day of sampling (Fig. 1B). The baseline concentration upstream is also generally higher than the following two days of sampling. This is attributed to a rainfall event the day before, on Friday, causing water levels in the creek to rise (data source: United States Geological Survey) and likely re-suspending sediment (Fig. S4†).
Estimates were made for various parameters associated with bathing in the creek and estimated oxybenzone concentrations were compared with the measured concentrations of oxybenzone. In order to arrive at units of oxybenzone concentration, the number of bathers, sunscreen used per bather,24 and fraction of sunscreen wash-off25 were varied across one order of magnitude, which allowed calculation (eqn (1)) of a range of possible oxybenzone concentrations (Table 1).
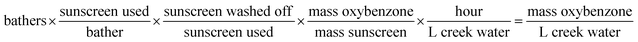 | (1) |
| Bathers (number) | Sunscreen used/bather (g) | Sunscreen wash-off (fraction) | Modeled [oxybenzone]in creek (ng L−1) |
|---|---|---|---|
| 30 | 0.3 | 0.1 | 4 |
| 30 | 1 | 0.1 | 12 |
| 30 | 3 | 0.1 | 37 |
| 30 | 0.3 | 0.3 | 11 |
| 30 | 1 | 0.3 | 37 |
| 30 | 3 | 0.3 | 110 |
| 100 | 0.3 | 0.1 | 12 |
| 100 | 1 | 0.1 | 41 |
| 100 | 3 | 0.1 | 123 |
| 100 | 0.3 | 0.3 | 37 |
| 100 | 1 | 0.3 | 123 |
| 100 | 3 | 0.3 | 368 |
| 300 | 0.3 | 0.1 | 37 |
| 300 | 1 | 0.1 | 123 |
| 300 | 3 | 0.1 | 368 |
| 300 | 0.3 | 0.3 | 110 |
| 300 | 1 | 0.3 | 368 |
| 300 | 3 | 0.3 | 1104 |
Many of the resulting calculated concentrations were within the range of [oxybenzone] measured. What we consider to be reasonable estimates for each parameter (∼100 bathers in the creek for any given time-averaged hour, 1 g average sunscreen applied per bather, and 30% sunscreen wash-off) gives a calculated [oxybenzone] of 122 ng L−1, squarely in the range of the measured concentrations. Future work on this system will include refined estimates of these parameters.
Given the modeled oxybenzone concentrations in Table 1, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 TiO2
10 TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxybenzone ratio, we expect that low and high concentrations of TiO2 release in this recreational water range from 0.4 to 110 ng L−1.
oxybenzone ratio, we expect that low and high concentrations of TiO2 release in this recreational water range from 0.4 to 110 ng L−1.
Concurrent increase of aluminum and iron concentrations
The re-suspension of creek bed sediment would cause an increase in concentration of other naturally occurring elements, such as aluminum and iron. Prior experiments in Clear Creek show that the elements of interest (Ti, Al, Fe) are non-detectable in the dissolved fraction and thus the measured concentrations in this study for unfiltered samples are representative of suspended particles only. An increase in these elements was in fact observed, as seen in Fig. 2. Although aluminum is often present as a coating on TiO2 NPs used in sunscreen to prevent photochemical oxygen radical production,26 an increase of ∼100 μg L−1 Al above background was observed during afternoon recreational use, much larger than what would be expected from sunscreen release given that Al content is typically <5% of a sunscreen TiO2 NP and Ti only increased by ∼10 μg L−1.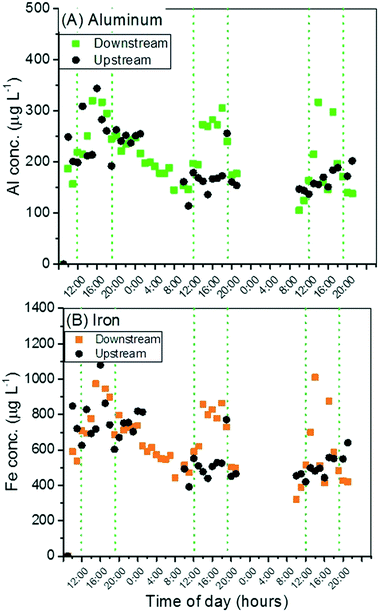 | ||
| Fig. 2 Al (A) and Fe (B) concentrations measured upstream and downstream over the three-day weekend. Peaks concurrent with those observed for Ti are also seen for Al and Fe. | ||
Previous work on TiO2 released from sunscreens into surface waters measured the ratio of Ti to other elements in suspended particulate matter over the course of a year to attempt differentiation of anthropogenic Ti from natural Ti sources.11 Using this approach in the present study, the results for Ti/Al are shown in Fig. 3. An increase in this ratio does occur concurrently with recreational activity in the creek, and suggests the input of anthropogenic Ti. The ratio of Ti/Fe is also plotted in Fig. 3, and follows the same diurnal pattern. The ratio of Al/Fe was also plotted, in Fig. S5,† and remains stable over the course of the sampling period during both peak and off-peak times, suggesting a similarity in mineralogy between the already-suspended sediments and those sediments re-suspended by human activity. The stability of this mineralogical ratio further suggests that any variations in the Ti/Al and Ti/Fe ratios may be due to anthropogenic inputs of Ti. However, the alternative is that the bed sediment re-suspended by bathers has greater titanium content than the natural suspended sediments. Further work on sediment characterization is required to ascertain the relative Ti, Al, and Fe content.
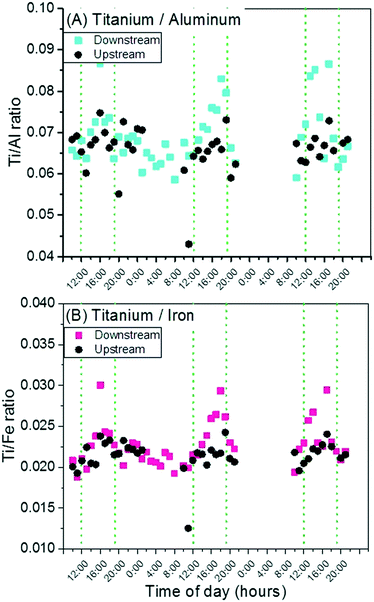 | ||
| Fig. 3 Ratios of Ti to Al (A) and Ti to Fe (B). Each ratio is higher downstream than upstream during peak use hours. | ||
A sensitivity analysis was performed to investigate how much more oxybenzone would need to be added to the creek in order to match the observed amount of [Ti] increase at the downstream site during bathing, if the oxybenzone and TiO2 were both at the same concentration in the sunscreen (commonly 5%). This involves assumptions which may not be completely true, such as: oxybenzone does not degrade between the time it is released until it is sampled and measured, and that TiO2 NPs and oxybenzone behave identically in terms of wash off from skin, diffusion in the water, and partition in solid-water phases. It was beyond the scope of this study to test these assumptions. Fig. S6† shows 1×, 10×, and 20× [oxybenzone] downstream additions to the upstream [Ti] and divided by the downstream [Fe], using the following equation:
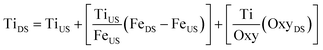 | (2) |
In this mass balance we assume that the concentration of Ti at the downstream site is a result of the Ti being transported from the upstream site, plus that which is resuspended (obtained from the amount of Fe resuspended and the Ti/Fe ratio in the sediment), and that coming from sunscreen (obtained from the oxybenzone introduced by the swimmers and a factor relating to the relative amount of oxybenzone-based sunscreen used compared to titanium-based sunscreen). An informal local market search found that the availability of TiO2 – based sunscreens compared to oxybenzone – based sunscreens was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Both types of products contain roughly 5% to 15% by mass of either active sunblock agent. Holding the ratio of TiO2
10. Both types of products contain roughly 5% to 15% by mass of either active sunblock agent. Holding the ratio of TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxybenzone constant at 1
oxybenzone constant at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in eqn (2) allows the determination of different Ti sources to Clear Creek (Fig. 4A).
10 in eqn (2) allows the determination of different Ti sources to Clear Creek (Fig. 4A).
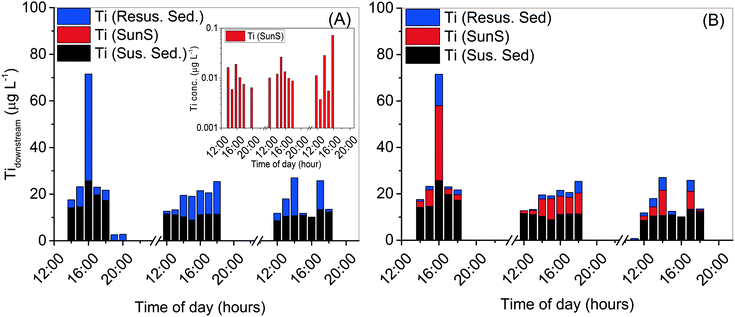 | ||
Fig. 4 Calculated contributions of titanium, from different sources of titanium input to Clear Creek, calculated from eqn (2) when: A) titanium/oxybenzone ratio is held constant at Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Oxy = 1 Oxy = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with inset for Ti(SunS) values; and B) when titanium/aluminum ratio is held constant at average upstream values of Ti 10 with inset for Ti(SunS) values; and B) when titanium/aluminum ratio is held constant at average upstream values of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 0.067. Al = 0.067. | ||
At this TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxybenzone ratio, it is likely that interference from natural Ti – containing particles (water column suspended sediments and resuspended bed sediments) masks any contribution from engineered TiO2 released from sunscreen.
oxybenzone ratio, it is likely that interference from natural Ti – containing particles (water column suspended sediments and resuspended bed sediments) masks any contribution from engineered TiO2 released from sunscreen.
The inset in Fig. 4A shows the contribution of sunscreen Ti to total measured Ti, calculated using measured [Ti] and eqn (2) to be in the range of 4 to 72 ng L−1 Ti (7–120 ng L−1 as TiO2). These values are quite close to those predicted in the environment by previous modeling studies,1–3 and also in agreement with the values based on oxybenzone measurement and TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxybenzone ratio in commercially available sunscreen (Table 1).
oxybenzone ratio in commercially available sunscreen (Table 1).
Fig. 4B shows the scenario where the Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio is held constant at a representative suspended sediment upstream value of 0.067. When this is the case, much more of the observed downstream Ti increase must be attributed to engineered TiO2 released from sunscreen, although the computed amount appears unrealistically high based on the measured oxybenzone concentration and likely usage patterns of sunscreen (proportions of TiO2vs. oxybenzone as active ingredient). Fig. 5 shows the Ti
Al ratio is held constant at a representative suspended sediment upstream value of 0.067. When this is the case, much more of the observed downstream Ti increase must be attributed to engineered TiO2 released from sunscreen, although the computed amount appears unrealistically high based on the measured oxybenzone concentration and likely usage patterns of sunscreen (proportions of TiO2vs. oxybenzone as active ingredient). Fig. 5 shows the Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al and Ti
Al and Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratios required for [Ti]downstream to be equivalent to the [Ti] calculated from eqn (2) when the TiO2
Fe ratios required for [Ti]downstream to be equivalent to the [Ti] calculated from eqn (2) when the TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxybenzone ratio = 1
oxybenzone ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, a more realistic ratio. It is possible that some of the observed [Ti] increase is in fact due to engineered TiO2 NP release due to bathing. Certainly, some of the Ti is also due to natural Ti-containing particles as well. The contribution of Ti from suspended sediments from upstream sources was anticipated but the impact of human-caused bed sediment resuspension was not. Future work will aim to develop methods for differentiation of natural and engineered particles using spICP-MS primarily. Additionally, future work on sunscreen release in this system will incorporate surveys for bathers to ascertain the type (mineral or organic) and quantity of sunscreen used.
10, a more realistic ratio. It is possible that some of the observed [Ti] increase is in fact due to engineered TiO2 NP release due to bathing. Certainly, some of the Ti is also due to natural Ti-containing particles as well. The contribution of Ti from suspended sediments from upstream sources was anticipated but the impact of human-caused bed sediment resuspension was not. Future work will aim to develop methods for differentiation of natural and engineered particles using spICP-MS primarily. Additionally, future work on sunscreen release in this system will incorporate surveys for bathers to ascertain the type (mineral or organic) and quantity of sunscreen used.
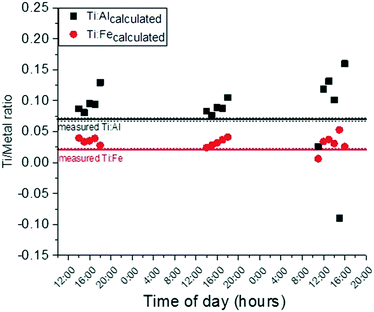 | ||
Fig. 5 Titanium to aluminum and/or iron ratio required for titanium downstream concentration to be equivalent to calculated titanium concentrations (eqn (2)) if Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxybenzone ratio is held constant at 1 oxybenzone ratio is held constant at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Solid lines are the ratios of Ti to Al and Fe for the upstream suspended sediments. Dotted lines are ± one standard deviation from the mean. 10. Solid lines are the ratios of Ti to Al and Fe for the upstream suspended sediments. Dotted lines are ± one standard deviation from the mean. | ||
Single particle ICP-MS measurement of titanium does not show diurnal or size trends
Fig. S7† shows representative time-resolved spICP-MS data from downstream and upstream samples. Ti-containing particle number concentrations were quantified by counting the number of pulses that were three standard deviations above the mean background signal. This showed no noticeable increase during peak recreation times.Selected water samples were measured by spICP-MS to look for differences in the signal distribution between upstream samples, downstream peak samples, and downstream off-peak samples. It was hypothesized that, although much of the difference in Ti concentrations may be due to re-suspension of large natural particles from the creek bed, engineered particles will be present as NPs which generate less intense signals close to the size limit of what a quadrupole instrument run in spICP-MS mode can detect. The time-resolved spICP-MS data for five downstream, two upstream, and one DI blank water samples analyzed for 49Ti were binned for frequency counts (example of binned data shown in Fig. S8†). In order to test for differences between samples in the lowest signal intensity range (corresponding to the smallest size particles), the lowest 15 instrument signals (0–14 counts) were each divided by the total number of signals in the 0–14 count range and are shown in Fig. S9† as the percent of each signal measured in each bin. No observable trends between upstream samples, downstream peak samples, and downstream off-peak samples were observed. This is likely due to a very broad distribution in the amount of Ti contained in a given particle, whether natural or engineered. Even if engineered NPs present in sunscreen are in fact being detected, there are likely too many background natural Ti-containing particles of all sizes present for any trends in instrument signal intensity to be observed. The size detection limit for TiO2 using a quadrupole ICP-MS was measured by Lee et al. to be ∼91 nm in DI water.27 Although this will vary from instrument to instrument, it is generally representative of the minimum diameter detectable for TiO2.
High resolution single particle ICP-MS provides improved size detection limit for TiO2
The main reasons that detection of TiO2 NPs by quadrupole spICP-MS is limited are interferences on the most abundant isotope, 48Ti. Isobaric interferences from 48Ca and 32S16O are both detected together with 48Ti when using a quadrupole ICP-MS with 1 atomic mass unit resolution. A potential avenue for reduction of the size detection limit, and future environmental analysis, is to use high resolution spICP-MS (HR-spICP-MS). This instrument can resolve signals at 0.01 atomic mass units, allowing differentiation of 48Ti from Ca and SO interferences and enabling use of the much more abundant (73.7%) 48Ti isotope. This allows a roughly tenfold increase in signal for a given mass of Ti relative to 49Ti or 47Ti. Since the instrument signal is proportional to the particle mass, and particle mass is proportional to the radius cubed, a tenfold increase in particle signal for a particle results in a 2.15-fold (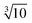 ) reduction in diameter.
) reduction in diameter.
An investigation of the size detection limit for the quadrupole instrument used in this study showed that we can detect a mass of Ti equivalent to a 79 nm TiO2 particle in Clear Creek water, using a cut-off three standard deviations above the mean background signal. With this cut-off, we are likely to miss detecting a large portion of TiO2 NPs released from sunscreen, as many of these may be present as smaller particles.28 A Ti calibration curve (Fig. S10†) and select Clear Creek samples were analyzed for 48Ti by HR-spICP-MS in an effort to reduce the size detection limit for TiO2. The minimum size detectable three standard deviations above background was measured to be 42 nm (Table S1†), a large improvement over the quadrupole instrument. This was investigated as a proof of concept for TiO2 NP detection in this case, and not yet applied to a full suite of water samples. We plan to apply HR-spICP-MS analysis to research on TiO2 NPs in surface water samples during future sampling campaigns.
Conclusions
This work demonstrates the challenges involved in detecting and quantifying release of engineered TiO2 NPs in natural waters. Although trace organics such as oxybenzone help us confirm that sunscreen constituents are being released, the large natural background of Ti and likely human-caused re-suspension of natural Ti-containing particles, possibly having metal ratios different than the suspended sediments, makes certain quantification of TiO2 NPs difficult. Modeled TiO2 release concentrations in the range of 0.4 to 110 ng L−1 are in agreement with surface water concentrations predicted in the environment,1–3 but the measured increase in [Ti] during recreation is in the tens of μg L−1, likely as a result of sediment resuspension. Another commonly used mineral sunscreen agent is ZnO, but analysis of Zn/Fe ratios (Fig. S11†) demonstrated no diurnal trends, possibly due to the high levels of Zn resulting from abandoned metal mines located in the upper reaches of the watershed. The use of element ratios can help detect a minute increase of one element in the presence of natural particles over weekly time scales,11 but may not always be sensitive enough for a diurnal study. Single particle ICP-MS is a promising technique for the detection of many NPs in environmental samples, but single-element monitoring by this technique is often not sufficient for differentiation of engineered from natural particles. Multi-element detection in each discrete particle by spICP-MS is promising for future environmental analysis,18 and high resolution mass spectrometry can help reduce the size detection limit of NPs as demonstrated here. This study demonstrates that human activities including recreation can have significant impacts on water quality in numerous ways, and compounds used in personal care products (oxybenzone, TiO2) can reach significant levels.Decades of instrument development with HPLC, LC-MS-MS, and GC-MS-MS has allowed environmental chemists to detect pollutants at ng L−1 levels and differentiate molecules to incredibly high resolution. This has advanced our understanding of environmental fate and transport processes as well as biogeochemical transformations. Further advancements in detecting and differentiating engineered from natural nano-scale colloids will likely provide unforeseen benefits to understand both natural ecosystems and new biogeochemical mechanisms involving nano-scale materials.
Acknowledgements
We are very grateful to Mickaël Tharaud and Marc Benedetti at the Institute de Physique du Globe de Paris for help with analysis of creek samples by high resolution ICP-MS. Partial funding was provided from the US Environmental Protection Agency through the STAR program (RD83558001) and the Network for the Lifecycle of Nanomaterials (LCNano). The use of trade, product, or firm names in this report is for descriptive purposes only and does not imply endorsement by the U.S. Government. The tests described and the resulting data presented herein, unless otherwise noted, were obtained from research conducted under the Environmental Quality Technology Program of the United States Army Corps of Engineers by the USAERDC. Permission was granted by the Chief of Engineers to publish this information. The findings of this report are not to be construed as an official Department of the Army position unless so designated by other authorized documents.Notes and references
- F. Gottschalk and B. Nowack, J. Environ. Monit., 2011, 13, 1145–1155 Search PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 Search PubMed.
- B. Nowack and N. C. Mueller, EMPA Act, 2008, vol. 41(2008–2009), p. 63 Search PubMed.
- L. Geranio, M. Heuberger and B. Nowack, Environ. Sci. Technol., 2009, 43, 8113–8118 Search PubMed.
- J. Farkas, H. Peter, P. Christian, J. A. Gallego Urrea, M. Hassellöv, J. Tuoriniemi, S. Gustafsson, E. Olsson, K. Hylland and K. V. Thomas, Environ. Int., 2011, 37, 1057–1062 Search PubMed.
- L. Windler, C. Lorenz, N. Von Goetz, K. Hungerbuhler, M. Amberg, M. Heuberger and B. Nowack, Environ. Sci. Technol., 2012, 46, 8181–8188 Search PubMed.
- C. Lorenz, L. Windler, N. von Goetz, R. P. Lehmann, M. Schuppler, K. Hungerbühler, M. Heuberger and B. Nowack, Chemosphere, 2012, 89, 817–824 Search PubMed.
- D. M. Mitrano, E. Rimmele, A. Wichser, R. Erni, M. Height and B. Nowack, ACS Nano, 2014, 8, 7208–7219 Search PubMed.
- X. Bi, R. Reed and P. Westerhoff, Characterization of nanomaterials in complex environmental and biological media, 2015 Search PubMed.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Pérez-Rivera and K. Hristovski, Environ. Sci. Technol., 2009, 43, 6757–6763 Search PubMed.
- A. P. Gondikas, F. v. d. Kammer, R. B. Reed, S. Wagner, J. F. Ranville and T. Hofmann, Environ. Sci. Technol., 2014, 48, 5415–5422 Search PubMed.
- R. D. Holbrook, D. Motabar, O. Quinones, B. Stanford, B. Vanderford and D. Moss, Environ. Pollut., 2013, 181, 68–74 CrossRef PubMed.
- H. E. Pace, N. J. Rogers, C. Jarolimek, V. A. Coleman, C. P. Higgins and J. F. Ranville, Anal. Chem., 2011, 83, 9361–9369 Search PubMed.
- D. M. Mitrano, A. Barber, A. Bednar, P. Westerhoff, C. P. Higgins and J. F. Ranville, J. Anal. At. Spectrom., 2012, 27, 1131–1142 RSC.
- R. B. Reed, D. G. Goodwin, K. L. Marsh, S. S. Capracotta, C. P. Higgins, D. H. Fairbrother and J. F. Ranville, Environ. Sci.: Processes Impacts, 2013, 15, 204–213 Search PubMed.
- L. D. Scanlan, R. B. Reed, A. V. Loguinov, P. Antczak, A. Tagmount, S. Aloni, D. T. Nowinski, P. Luong, C. Tran, N. Karunaratne, D. Pham, X. X. Lin, F. Falciani, C. P. Higgins, J. F. Ranville, C. D. Vulpe and B. Gilbert, ACS Nano, 2013, 7(12), 10681–10694 Search PubMed.
- R. B. Reed, C. P. Higgins, P. Westerhoff, S. Tadjiki and J. F. Ranville, J. Anal. At. Spectrom., 2012, 27, 1093–1100 Search PubMed.
- O. Borovinskaya, B. Hattendorf, M. Tanner, S. Gschwind and D. Günther, J. Anal. At. Spectrom., 2013, 28, 226–233 Search PubMed.
- M. D. Montaño, H. R. Badiei, S. Bazargan and J. Ranville, Environ. Sci.: Nano, 2014, 1, 338–346 Search PubMed.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS PubMed.
- P. Westerhoff, Y. Yoon, S. Snyder and E. Wert, Environ. Sci. Technol., 2005, 39, 6649–6663 CrossRef CAS PubMed.
- A. M. Peck, Anal. Bioanal. Chem., 2006, 386, 907–939 Search PubMed.
- United States Environmental Protection Agency, SW-846 methods for the analysis of hazardous waste, 2007 Search PubMed.
- R. Neale, G. Williams and A. Green, Arch. Dermatol., 2002, 138, 1319–1325 Search PubMed.
- R. Stokes and B. Diffey, Br. J. Dermatol., 1999, 140, 259–263 CrossRef CAS PubMed.
- J. Virkutyte, S. R. Al-Abed and D. D. Dionysiou, Chem. Eng. J., 2012, 191, 95–103 Search PubMed.
- S. Lee, X. Bi, R. B. Reed, J. F. Ranville, P. Herckes and P. Westerhoff, Environ. Sci. Technol., 2014, 48, 10291–10300 Search PubMed.
- C. Botta, J. Labille, M. Auffan, D. Borschneck, H. Miche, M. Cabié, A. Masion, J. Rose and J.-Y. Bottero, Environ. Pollut., 2011, 159, 1543–1550 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: ESI includes photos of the sampling campaign, including the ISCO sampler used downstream of recreational use, and representative photos of Clear Creek near the downstream sampling site. Creek flow rate over the sampling weekend from United States Geological Society data, plotted with [Ti] upstream and downstream of recreational usage. The Fe/Al ratio in water samples collected during the weekend. A sensitivity analysis for addition of downstream oxybenzone concentrations times varying multipliers, added to upstream Ti concentrations, with the sum divided by the upstream Fe concentrations. Representative time-resolved spICP-MS data for Ti in creek samples, and Ti-containing particle number concentrations from spICP-MS analysis. The ratio of Zn/Fe, both upstream and downstream of recreational creek usage. See DOI: 10.1039/c6en00283h |
| This journal is © The Royal Society of Chemistry 2017 |