 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Refining tools to bridge the gap between academia and chemical regulation: perspectives for WikiREACH†
Marlene
Ågerstrand
 *a,
Mattheus
Brenig
*a,
Mattheus
Brenig
 b,
Martin
Führ
c and
Julian
Schenten
b,
Martin
Führ
c and
Julian
Schenten
 c
c
aDepartment of Environmental Science and Analytical Chemistry (ACES), Stockholm University, SE-106 91 Stockholm, Sweden. E-mail: marlene.agerstrand@aces.su.se; Tel: +46 8 16 40 21
bChair of Economic Policy and SME Research, University of Göttingen, 37073 Göttingen, Germany
cSociety for Institutional Analysis (sofia), Darmstadt University of Applied Sciences, 64295 Darmstadt, Germany
First published on 22nd November 2017
Abstract
Regulatory hazard and risk assessments of chemical substances have to include all reliable and relevant data to be credible and complete. However, screening the literature for appropriate studies and extracting data is burdensome. Therefore, reducing impediments by making data easily and readily accessible to risk assessors could result in more comprehensive hazard and risk assessments. In this paper, we study WikiPharma, a database that aggregates ecotoxicity data for pharmaceuticals, extracted from peer-reviewed studies. The use of the WikiPharma database is explored to develop strategies on how similar tools can bridge between science and policy by providing risk assessors with easily accessible summary data. Specifically, adapting the concept of WikiPharma to industrial chemicals regulated under the REACH regulation is discussed. Experiences with WikiPharma show that there is interest in using peer-reviewed studies in regulatory decision-making. However, tools like WikiPharma require constant updates. Hence, as for “WikiREACH”, effective incentives are needed to motivate researchers to feed in relevant data for regulatory assessments. Besides, support by automated processes can aid in the labour-intensive activity of gathering data. To ensure that such a tool is continuously maintained and compatible with the regulatory system, and thereby useful for hazard and risk assessments of chemicals, it would benefit from being developed in collaboration with the major stakeholders in the field, i.e. regulatory agencies, academia, industry, scientific journals, and providers of research network platforms.
Environmental significanceThis paper addresses how scientific research is (not) used in decision-making, focusing on peer-reviewed toxicity and ecotoxicity studies for regulatory assessments of chemicals. One possible reason for the overall low regulatory use of peer-reviewed studies is that the process of searching for studies is resource demanding. As a consequence, regulatory decisions are not based on all available information. This paper suggests the development of a tool that can facilitate the use of peer-reviewed studies in the REACH context (“WikiREACH”), based on experiences from the use and development of the WikiPharma database for ecotoxicity studies for pharmaceuticals. Creating equivalent tools for other groups of chemicals is possible and an important way forward. |
1 Introduction
There are several reasons why peer-reviewed studies, published i.a. by academic researchers, should be used in hazard and risk assessments of chemicals. First, to make the most appropriate decision on how to deal with hazards and risks of chemicals, all relevant information has to be considered. Second, to ignore peer-reviewed studies would be a waste of the usually publicly provided economic resources used when producing the studies. There are also additional ethical or moral considerations to take into account when research uses test animals. Third, peer-reviewed studies may use test designs, test species and test endpoints that have proven to be more sensitive and relevant than those used in standard studies usually performed by a professional laboratory on behalf of, or by, the producer/importer of chemicals, i.e. non-standard studies can be an important complement to the standard studies. Examples of chemical groups where this particularly applies are endocrine disrupting chemicals, pharmaceuticals, and nanomaterials.1–5Consequently, major sources of EU law require the use of all available data (see Table 1). This includes industry study reports and grey literature, as well as studies published in the peer-reviewed literature. However, peer-reviewed studies are not used to the extent that they could be in regulatory assessments.6 One reason is that the reporting used in peer-reviewed studies does not always fully meet regulatory requirements.7,8 Another reason for not using peer-reviewed studies in regulatory assessments is that searching for, and getting access to, studies is resource demanding.
| Source of law | Specific reference | Quote from legal text |
|---|---|---|
| Regulation no. 1907/2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) | Art. 12(1) | The registration dossier “shall include […] all physicochemical, toxicological and ecotoxicological information that is relevant and available to the registrant and as a minimum the following” [standard information requirement as defined in Annexes VI–IX] |
| Annex VII, last sentence | “Any other relevant physicochemical, toxicological and ecotoxicological information that is available shall be provided” | |
| Regulation no. 1272/2008 on classification, labelling and packaging of substances and mixtures (CLP) | Art. 5(1) | “Manufacturers, importers and downstream users of a substance shall identify the relevant available information for the purposes of determining whether the substance entails a physical, health or environmental hazard as set out in Annex I, and, in particular, the following” […] |
| Art. 6(1) | “Manufacturers, importers and downstream users of a mixture shall identify the relevant available information on the mixture itself or the substances contained in it for the purposes of determining whether the mixture entails a physical, health or environmental hazard as set out in Annex I, and, in particular, the following” […] | |
| Regulation no. 1107/2009 concerning the placing of plant protection products on the market | Art. 8(5) | The summary dossier added to any application for the approval of an active substance shall include “Scientific peer-reviewed open literature, as determined by the Authority, on the active substance and its relevant metabolites dealing with side-effects on health, the environment and non-target species and published within the last 10 years before the date of submission of the dossier shall be added by the applicant to the dossier” |
| Commission Regulation no. 283/2013 setting out the data requirements for active substances, in accordance with Regulation no. 1107/2009 | Annex, Section 5 | “All available biological data and information relevant to the assessment of the toxicological profile of the active, substance tested, including modelling, shall be reported” |
| Annex, Section 8 | “All available biological data and information which is relevant to the assessment of the ecotoxicological profile of the active substance shall be reported” | |
| Regulation no. 1223/2009 on cosmetic products | Art. 10(1) | “In order to demonstrate that a cosmetic product complies with Article 3, the responsible person shall, prior to placing a cosmetic product on the market, ensure that the cosmetic product has undergone a safety assessment on the basis of the relevant information and that a cosmetic product safety report is set up in accordance with Annex I” |
| Annex I | “The cosmetic product safety report shall, as a minimum, contain the following” […] “All available data on the undesirable effects and serious undesirable effects to the cosmetic product or, where relevant, other cosmetic products” |
In this paper, the focus is on the latter reason and possible ways to reduce the resources needed when gathering data, and thereby increase the use of peer-reviewed studies in hazard and risk assessment of chemicals. In a first step, the use and management of the WikiPharma database will be evaluated (Section 3). WikiPharma bridges the gap between peer-reviewed ecotoxicity studies and pharmaceutical regulation by making summaries of environmental effect data for pharmaceuticals freely available and easily accessible. Based on the findings from WikiPharma and available literature, ways to reduce the burden of using peer-reviewed studies in the context of REACH are explored (Section 4). REACH is the major EU regulatory scheme for chemical substances. It is a prime example of modern legislation, shifting responsibilities from public authorities to companies that manufacture, import, or use chemical substances.9 In particular, industry actors may have to submit a registration dossier to the European Chemicals Agency (ECHA) which contains “all physicochemical, toxicological and ecotoxicological information that is relevant and available to the registrant”.10,11
When filing a registration dossier with ECHA, registrants may need to conduct original research or search for already existing studies. In the latter case, registrants can search for information on the properties of chemicals used, in particular the database(s) of the OECD eChemPortal. It combines information prepared for several government chemical review programmes. However, to the authors' knowledge, it is not designed to facilitate access to peer-reviewed studies.
According to ECHA's 2016 evaluation, out of the 5% of registration dossiers per tonnage band subject to inspection by ECHA, 168 out of 184 (i.e. 91%) were non-compliant.12 This paper assumes that facilitated use of peer-reviewed studies can contribute to improved registration dossiers.
2 Method
Usage data from WikiPharma's Google Analytics were collected and evaluated together with information gained through correspondence with users of WikiPharma. Based on this information, a questionnaire was designed to further substantiate the analysis of the use and impact of WikiPharma. The questionnaire addressed awareness, use, usefulness, and motivation for using WikiPharma, as well as use of peer-reviewed studies in regulatory assessments, and opportunities for further developments of WikiPharma and similar tools (see ESI† for the questionnaire and the respondents' replies). With regard to each question, respondents were asked to pick pre-defined answers (multiple answers possible for some questions) and/or provide comments. In May and June 2017, 137 individuals from different sectors (academia, regulatory agency, industry, consultancy, others) were emailed a link to access the online questionnaire. The selection of addressees was based on “known users”, i.e. persons that corresponded with the developer, and a substantially larger group of “possible users”, i.e. people within the developers' extended network. The group “possible users” was included to increase the possibility of reaching also unknown users with information potentially important to us. Due to the generous selection of “possible users” a low response rate to the questionnaire was expected. Until July 10th, after two e-mail reminders, 30 individuals completed the questionnaire, yielding a response rate of 22%. Not all of them answered all questions.3 The WikiPharma database
In 2007–2010 an evaluation of the industry-owned voluntary environmental classification system for pharmaceuticals in Sweden (available at https://www.fass.se) was performed.13,14 In this process, peer-reviewed ecotoxicity studies for pharmaceutical substances were gathered in a database to facilitate comparisons with the data selection made by pharmaceutical companies participating in the classification system. One of the conclusions from the evaluation was that not all peer-reviewed ecotoxicity studies were used when classifying pharmaceuticals, and that no justifications for the exclusions were provided. This is not the only example of exclusion of peer-reviewed studies in risk and hazard assessments of chemicals. Previous research shows that the use of peer-reviewed studies as decision support may result in controversies, mainly because of disagreements as to the adequacy of studies.15–18 There are several possible reasons for not using peer-reviewed studies in the assessment of chemicals; one being that searching for studies in the scientific literature is labour intensive and access to studies may not be free of charge. To aid in this process, the database containing peer-reviewed ecotoxicity studies for human pharmaceuticals was made public in 2009 as part of the research programme MistraPharma (https://www.mistrapharma.se). The database is called WikiPharma, made available at https://www.wikipharma.org,19 and hosted by Stockholm University. The aim of WikiPharma is to provide an easily accessible overview of effects caused by pharmaceuticals on non-target organisms. Besides being a tool for risk assessors when assessing potential risks, WikiPharma can be used to identify data gaps in the scientific literature as well as regulatory assessments. Intended users are risk assessors from governmental agencies, consultancy firms, and industry, as well as researchers within the field of pharmaceuticals in the environment. WikiPharma provides data such as test methods, endpoints tested, exposure time, effect values, and bibliographic references to the corresponding publications. Only peer-reviewed studies are included. The reliability of included studies is not evaluated; this is instead done as an integrated step in the hazard or risk assessment. WikiPharma was constructed as a “wiki”, meaning that users can add data, to enable continuous updating of the database.3.1 The use and users of WikiPharma
Google Analytics shows that during 2016 WikiPharma had 4,295 visits by either new or returning visitors, in total there were 6,857 visits between September 2015 when Google Analytics was added to WikiPharma and April 2017 when this study was initiated. The visits represent 114 countries (see Fig. 1 for visits by country in percent). The number of visitors during 2016 was 3,043, 70% of whom were new visitors. However, this number may be misleading since data from Google Analytics are only available from September 2015. Therefore, visitors in 2016 who last visited the website before September 2015 are falsely classified as new visitors. This error becomes more likely with decreasing frequency of visits per visitor. Looking at the questionnaire, seventeen respondents answered that they visit WikiPharma less often than once a month. This indicates that the number of new visitors in 2016 may be overestimated. Furthermore, Google Analytics identifies returning visitors based on HTTP cookies stored on the client side. If users delete cookies, the error increases.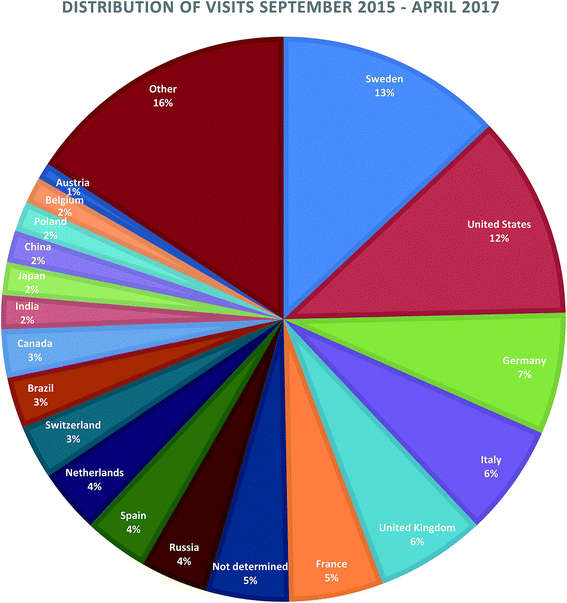 | ||
| Fig. 1 Top countries visiting WikiPharma.org between September 2015 and April 2017. In total 6.857 visits. | ||
It is difficult to say whether WikiPharma's 4,295 visits during 2016 are high or low in number. Analytics information for other databases is lacking and comparisons with other fields can be misleading since they may be different in size and scope. The number of people working with pharmaceuticals in the environment is low compared to other areas within chemical management and research (e.g. pesticides). Therefore, a qualitative overview of the users of the database may give a better impression of how widely the database is used by its target group. Correspondence with the developers of WikiPharma indicates that risk assessors working at consultancy firms and governmental agencies in Europe and North America primarily use the database. The following agencies have contacted the developers of WikiPharma and/or used the database in their work: US Food and Drug Administration (FDA); Health Canada; Delaware River Basin Commission (DRBC); Dutch National Institute for Public Health and the Environment (RIVM); Swedish Medical Product Agency; Stockholm County Council (SLL); German Environment Agency (UBA); and French Agency for Food, Environmental and Occupational Health & Safety (ANSES). The European network for regulators for Environmental Quality Standards within the Water Framework Directive has also used the database. This suggests that major national agencies in Europe with responsibility for pharmaceuticals in the environment are aware of the database. Consultancy firms and pharmaceutical companies located in the Netherlands, UK, Germany, Sweden, USA, Canada, and France have also used WikiPharma, as well as the European-based research network NORMAN. Altogether, it can be concluded that WikiPharma has been used in regulatory processes on several occasions in both Europe and North America. This was confirmed by the questionnaire, and almost half (thirteen of thirty) of the respondents were affiliated with regulatory agencies.
Correspondence indicates that the database is used as a source of information when preparing assessment dossiers and reports. Again, this is confirmed by the questionnaire. Asked for what purpose they have used WikiPharma, twenty-one respondents answered “As a resource when performing risk or hazard assessments”. Six of the respondents stated that they use it as a resource when performing laboratory research. The main reason for corresponding with the developers is to inform of a technical problem with the database, i.e. that the database is not accessible. Other, less frequent, inquiries concern typos and suggestions of smaller adjustments regarding the interface. Some also suggest new data by sending peer-reviewed studies directly, instead of using the wiki function.
Among the users corresponding with the developers there seems to be an overall positive attitude towards the database (citation from correspondence “This database is really the best in user-friendliness and completeness”). Presentations at international conferences and citations in scientific journals (twenty in total from 2010 to 2017, according to Scopus) show that the database is continuously used in the scientific community as a resource when describing the current state of knowledge. Seventeen of the respondents of the questionnaire confirmed that information from WikiPharma had been useful for their institutions (e.g. as a literature screening tool or for regulatory decisions), three negated this, and five answered that they did not know. Examples of comments accompanying the positive answers include: “When creating datasheets for pharmaceuticals to derive Environmental Quality Standards”, “As literature screening tool”, “Satisfy my need for ecotox data on emerging contaminants”, and “To decide which drug should be tested for its ecotoxicity”. Nineteen of the respondents of the questionnaire rated the scientific credibility of the intended WikiPharma approach (i.e. researchers add data to the database) as moderately high or high; seven rated it as moderately low or low. Commenting to another question, two respondents linked potential benefits of WikiPharma to measures of quality control (“It should pass a control-step before published”, and “If there is confirmation that data are valid”).
3.2 Limitations with WikiPharma, and possible improvements
According to the developers of WikiPharma, the main limitation of WikiPharma is that it has to be updated on a regular basis to be relevant for its users. This was anticipated already when developing the database, and therefore the wiki function was created giving users and, more specifically, researchers the opportunity to propose new data. In practice, until now, the wiki function has never been used. Thirteen of the respondents of the questionnaire stated that they, or the institution that they are affiliated with, produce studies relevant for WikiPharma. Only one of them claimed having added such data to the database. It is possible that this respondent emailed the study directly to the developers instead of adding it using the wiki function.There may be several reasons why the wiki function has not been used: low awareness of the existence of the database, including the possibility to suggest data; low awareness among academic researchers of the importance of making risk assessors aware of studies that can be used in regulatory assessment;20 labour-intensive to add studies to the database since a user account has to be created and data have to be added manually; and no direct benefits within the academic system, i.e. the impact measurement used (the h factor) only accounts for citations of peer-reviewed studies, even though contribution to a regulatory process may exceed the societal impact of a peer-reviewed study several times.21 Of the twelve respondents who have not added data to WikiPharma despite having produced studies relevant for WikiPharma, three stated having been unaware of the wiki function in WikiPharma as a reason. One respondent replied being unaware of the regulatory use of WikiPharma, one replied that it was too labour-intensive to add studies, one replied that there was no direct benefit to their institution to add data, and one was using another database.
Contrary to expectations, nineteen respondents saw benefits for researchers in adding data to WikiPharma, examples of comments include: “The data have a higher chance of becoming relevant for regulatory purposes”, “Provides valuable overview on relevant environmental toxicity data available for active pharmaceutical ingredients”, and “Increase use of their data”. When asked about what motivates/would motivate them to add data to WikiPharma, fourteen replied “Knowing it may have regulatory impact”, nine respondents replied “Request from funding agencies to communicate results outside of academia”, eight replied “A hyperlink that connects the provided data with publicly available literature databases hosting the article the data is retrieved from”, five replied “Knowing that it may increase citations”, four replied “Nothing”, and two replied “Additional scores by web tools such as ResearchGate”. When asked about the importance of increasing the awareness among risk assessors about peer-reviewed ecotoxicity studies that could be used in regulatory assessments twenty-four respondents answered that this is very or moderately important.
Since the wiki function has not been used, the WikiPharma database has depended on updates by the developers to continue to serve as a useful tool for risk assessors and researchers. The developers have updated the database on three occasions since 2009. Due to the, in comparison to other groups of chemicals, low number of ecotoxicity studies this work has been manageable. To make academic researchers suggest studies to the WikiPharma database a thorough information campaign targeting researchers working in the field of pharmaceuticals in the environment could be one option. However, gaining the attention of academic researchers is a difficult task if direct benefits cannot be offered in return. One way to incentivise researchers to contribute to regulatory processes is by considering societal relevance when allocating research funds. In fact, funding agencies are increasingly interested in the societal relevance of research proposals. For this purpose, measures of societal relevance are needed. The EU Horizon 2020 research programme Inspiration has developed indicators for societal relevance that can be used when evaluating research projects and proposals. Examples of indicators include cooperation with stakeholders, and a detailed presentation from the researchers of how and where research results can directly be implemented in policy.22 Incentives could also be raised if contributions were quantified in non-traditional impact measures like the ones calculated by ResearchGate or Altmetrics. Making the updating of studies to WikiPharma automatic through collaborations with scientific journals is another option which may facilitate the continuous use of the database.
Another limitation of the database is that it does not give direct access to the peer-reviewed studies from which data were gathered. If the peer-reviewed studies are not published with open access this may prevent risk assessors from using them. Access to studies could be provided by adding a function to WikiPharma that allows users to send a request to the corresponding author.
Suggestions from the respondents of the questionnaire on how WikiPharma could be improved include expanding the scope to also include environmental monitoring data and regulatory assessments, and adding a possibility to search the database by CAS-numbers. Another suggestion is to allow users to comment on the reliability of the studies so that users can learn from others assessments and use of the studies.
In the questionnaire, the respondents were asked if they saw a need to develop databases (or similar tools) for other groups of chemicals (e.g. pesticides, chemical substances under REACH, and cosmetic substances) to increase the use of peer-reviewed studies in regulatory assessments. Twelve of them agreed while two negated it, and eight answered that they did not know. Examples of comments from those that agreed include: “Data sharing is fundamental”, “There is an increasing interest in such data”, “Databases are needed for any type of contaminants”, “It would be very useful to have a database for industrial chemicals or ingredients of cosmetics”, “Could be relevant for other emerging issues, such as nanomaterials and microplastic”, and “Essential to complete existing information but should not duplicate existing tools”. Ideas for how such tools should be developed and designed included that the tools should be compatible with existing tools to allow information to be merged from different sources, to involve industry organizations in the process, and to have interactive platforms.
In conclusion, the analysis of WikiPharma has shown that (1) there is interest in using peer-reviewed studies in regulatory decision-making, (2) databases compiling data are appreciated tools that facilitate use of peer-reviewed studies in various processes, (3) tools compiling or listing data need to be constantly updated to be useful for risk assessors and researchers, and (4) tools should be compatible with current regulatory systems to be able to be utilized fully.
4 Exploring perspectives to promote the use of peer-reviewed studies in REACH
REACH requires registrants of chemical substances to take into account all physicochemical, toxicological and ecotoxicological information that is reliable, relevant, and available to them (see Table 1). However, in practice, registrants do not use available peer-reviewed studies to the extent that they could.6 To overcome this, researchers have to produce studies of sufficient reliability and relevance to regulatory decision-making,20 and risk assessors within industry and regulatory agencies have to search for and evaluate peer-reviewed studies for use in assessments of chemicals, and scientific journals have to support both researchers and risk assessors in this process.Adapting the WikiPharma approach to REACH could act as an enabler in this respect. Adding ecotoxicity studies to the WikiPharma database has been manageable since studies are available for a limited number of pharmaceuticals (currently less than 200 substances published in around 300 peer-reviewed studies). As for REACH chemicals, and other chemical groups such as pesticides, many more substances and studies have to be taken into account. This increases the burden of manually adding studies to the database. This also renders approaches unfeasible, where a single (regulatory) agency gathers data derived from the peer-reviewed literature, like in the case of the ECOTOX database hosted by the US EPA (https://www.cfpub.epa.gov/ecotox/). Just like WikiPharma it is free of charge and data are added manually (by a contractor to US EPA). In contrast to WikiPharma, data are only added for a limited number of substances that are being assessed, i.e. not for all substances within a specific chemical group. Such an approach does not seem to be viable for REACH. Indeed, one of the major motivations to implement REACH was that, under the preceding legislation, authorities were overwhelmed with the task of performing hazard and risk assessments for the ever-increasing amount of substances. The major accomplishment of REACH was, thus, to shift the burden of proof from authorities to industry.9,23 Taking this into account and considering the increased burden of manually adding studies to a database, it may be beneficial to automate the process to some extent. For this purpose, it would be appropriate to collaborate with scientific journals. Several publishers maintain central databases with bibliographic information on papers published in proprietary scientific journals, often linked to the full texts of these papers (e.g.http://www.sciencedirect.com, https://www.link.springer.com/and http://www.onlinelibrary.wiley.com/). Publishers or specific journals could e.g. provide access to selected data of these databases to regulators and risk assessors. To create such mechanisms, a dialog between actors in academia, industry, regulation, and scientific journals is necessary to ensure that needs and limitations are understood and considered. Getting scientific journals to collaborate may be an issue if they expect less profit from commercializing scientific research. However, scientific journals could still price access to full texts. Alternatively, journals could require authors to contribute to the database as part of the guidelines for submission. A similar route has been taken by the scientific journal RNA Biology in collaboration with other stakeholders. Authors submitting papers to the journal are required to write a Wikipedia entry that summarizes the research results.24
WikiPharma has been hosted by Stockholm University and this has had some limitations. First and foremost, since updating and maintaining the database is not a research activity, funding has been problematic. Second, the possibility of reaching a sufficient number and range of users has been limited to the developers' extended network. A suitable host for an equivalent REACH tool could for example be the European Commission, ECHA, or OECD. These actors are in the position to provide the resources needed to handle contacts with stakeholders as well as the required resources for development and maintenance. In addition, they have the necessary knowledge about what is needed to make such a tool compatible with the regulatory needs.
In the so-called dissemination portal ECHA publishes, free of charge, information on registered substances under the REACH regulation. One option is to add to this portal a “dashboard” where new information can be pinned on. Using standard identifiers such as CAS numbers, added information could get automatically assigned to the dossier of the substance in question. A specific search tool to be developed could (semi-)automatically retrieve substance data from the databases of scientific journals and transfer them to the dashboard. This function might as well be integrated in the OECD eChemPortal, also hosted by ECHA. In addition, researchers could be allowed to add data from peer-reviewed studies to the dashboard using a wiki approach (“WikiREACH”). Thereby, relevant data published in scientific journals that are not willing or not able to provide data could be added manually. Besides, establishing collaborations including all relevant actors might take some time. Adding the wiki function is therefore a means to promote the use of peer-reviewed data in a short-term perspective. REACH registrants and ECHA could be alerted when data are added to the dashboard. Whether or not to use these data is to be decided by these actors; however, registrants are legally obliged to update their registration without undue delay when relevant new information comes to their attention.
Various options to increase the use of the wiki function by researchers have been identified (Section 3.2). In addition, effective incentives are pivotal to create user motivation. One suggestion is that providers of research network platforms, such as ResearchGate.net or Academia.edu, could provide such incentives by introducing a bridge between science and regulation. In a nutshell, authors could be asked to, when adding new papers to the platform, answer a pre-defined set of questions that could be used to identify research results suitable for regulatory decision-making. The questions would concern CAS-number, substance name, substance group (e.g. industrial chemical, pesticide, pharmaceutical), type of data (e.g. ecotoxicity study or biomonitoring data), research results (e.g. effect values or measured environmental concentrations), etc. In addition, a link to the dashboard in the relevant regulatory database could be provided automatically. Questions like this have the potential to inspire researchers to consider the regulatory use of their research results.
The issue of quality control was also highlighted by respondents of the questionnaire. In WikiPharma the peer-review process is used as a quality control method, but this does not mean that the studies included in the database are considered to be of sufficient reliability for regulatory purposes. Studies would therefore still have to undergo regular evaluation before deciding whether the study can be included in an assessment. To proactively ensure studies of sufficient reliability, authors should be approached with guidance on how to reach reliable outcomes. For instance, in the pre-defined set of questions mentioned in the previous paragraph authors could be asked if, when performing the research documented in the article, they considered reliability requirements.20,25
5 Summary and conclusions
The WikiPharma database works as a bridge between science and policy. It provides easily accessible summary data of ecotoxicity studies for pharmaceuticals. The aggregation of information saves time, which makes it a tool appreciated by risk assessors and researchers. The database is continuously used in regulatory work as well as research, both in North America and Europe. The use of WikiPharma shows that there is interest in using peer-reviewed studies in regulatory decision-making, however to ensure future use of the database it has to be updated on a regular basis.Acknowledging that there is interest among risk assessors to use tools that simplify the search for relevant studies to be incorporated in regulatory assessments for pharmaceuticals, it can be assumed that this also holds true for other groups of chemicals. Options to increase awareness of and create incentives for researches to make use of tools like WikiREACH have been identified, including the involvement of research network platforms. The outlined approach yields the additional benefit of creating regulatory awareness among researchers, i.e. by encouraging researchers to adapt studies so that, in addition to providing results interesting for the research community, the studies would be adequate for regulatory hazard and risk assessments.
To ensure that the tool remains updated, an automatic selection and compilation of studies should be considered. Such a tool would benefit from being developed in collaboration with the major stakeholders in the field, i.e. academia, industry, regulatory agencies, scientific journals, and providers of research network platforms. This would ensure that the tool is compatible with the regulatory system and, thereby, used in hazard and risk assessments of chemicals. For chemical regulation to be credible and complete, assessments have to include all reliable and relevant data. A tool such as the outlined WikiREACH could be one way of facilitating that. To put the tool into practice without delay, and thus contribute to the normative aims, we suggest the following next steps:
(1) Collect feedback from regulatory agencies, i.e. ECHA in particular, on the technical options to create and maintain the dashboard.
(2) Further explore options to involve research network platforms.
(3) Initiate cooperation with scientific journals.
(4) Develop and test the tool, including the dashboard.
Conflicts of interest
Ågerstrand has developed and is managing the WikiPharma database.Acknowledgements
The German Federal Ministry for Education and Research (BMBF) funded this research as part of the research project KInChem (FKZ 01UT1419A-B). The WikiPharma database was developed using funding from the Swedish Research Council Formas.References
- M. Ågerstrand, et al., Improving Environmental Risk Assessment of Human Pharmaceuticals, Environ. Sci. Technol., 2015, 49, 5336–5345 CrossRef PubMed.
- EFSA Scientific Committee, Scientific Opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment, EFSA J., 2013, 11(3), 3132 CrossRef.
- N. B. Hartmann, M. Ågerstrand, H.-C. H. Lützhøft and A. Baun, NanoCRED: A transparent framework to assess the regulatory adequacy of ecotoxicity data for nanomaterials – Relevance and reliability revisited, NanoImpact, 2017, 6, 81–89 CrossRef.
- A. Kortenkamp, et al., State of the art assessment of endocrine disrupters, Final report, http://www.ec.europa.eu/environment/chemicals/endocrine/pdf/sota_edc_final_report.pdf, 2011 Search PubMed.
- A. Lillicrap, A. Macken and K. V. Thomas, Recommendations for the inclusion of targeted testing to improve the regulatory environmental risk assessment of veterinary medicines used in aquaculture, Environ. Int., 2015, 85, 1–4 CrossRef PubMed.
- E. Ingre-Khans, M. Ågerstrand, A. Beronius and C. Rudén, Transparency of Chemical Risk Assessment Data under REACH, Environ. Sci.: Processes Impacts, 2016, 18, 1508–1518 CAS.
- M. Ågerstrand, L. Edvardsson and C. Rudén, Bad Reporting or Bad Science? Systematic Data Evaluation as a Means to Improve the Use of Peer-Reviewed Studies in Risk Assessments of Chemicals, Hum. Ecol. Risk Assess. Int. J., 2013, 20, 1427–1445 CrossRef.
- M. Ågerstrand, M. Breitholtz and C. Rudén, Comparison of four different methods for reliability evaluation of ecotoxicity data: a case study of non-standard test data used in environmental risk assessments of pharmaceutical substances, Environ. Sci. Eur., 2011, 23, 17 CrossRef.
- M. Führ and K. Bizer, REACH as a paradigm shift in chemical policy – responsive regulation and behavioural models, J. Cleaner Prod., 2007, 15, 327–334 CrossRef.
- European Chemicals Agency, Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.4: Evaluation of available information, https://www.echa.europa.eu/documents/10162/13643/information_requirements_r4_en.pdf, 2011 Search PubMed.
- European Chemicals Agency, Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.3: Information gathering, https://www.echa.europa.eu/documents/10162/13643/information_requirements_r3_en.pdf, 2011 Search PubMed.
- European Chemicals Agency, Evaluation under REACH. Progress Report 2016. Evaluation in ECHA's Integrated Regulatory Strategy, https://www.echa.europa.eu/documents/10162/13628/evaluation_report_2016_en.pdf, 2016 Search PubMed.
- M. Ågerstrand, M. Wester and C. Rudén, The Swedish Environmental Classification and Information System for Pharmaceuticals — An empirical investigation of the motivations, intentions and expectations underlying its development and implementation, Environ. Int., 2009, 35, 778–786 CrossRef PubMed.
- M. Ågerstrand and C. Rudén, Evaluation of the accuracy and consistency of the Swedish Environmental Classification and Information System for pharmaceuticals, Sci. Total Environ., 2010, 408, 2327–2339 CrossRef PubMed.
- R. E. Alcock, B. H. MacGillivray and J. S. Busby, Understanding the mismatch between the demands of risk assessment and practice of scientists — The case of Deca-BDE, Environ. Int., 2011, 37, 216–225 CrossRef CAS PubMed.
- A. Beronius, C. Rudén, H. Håkansson and A. Hanberg, Risk to all or none?: A comparative analysis of controversies in the health risk assessment of Bisphenol A, Reprod. Toxicol., 2010, 29, 132–146 CrossRef CAS PubMed.
- M. D. Boone, et al., Pesticide Regulation amid the Influence of Industry, BioScience, 2014, 64(10), 917–922 CrossRef.
- C. J. Portier, B. K. Armstrong and B. C. Baguley, et al., Differences in the carcinogenic evaluation of glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA), J. Epidemiol. Community Health, 2016, 70, 741–745 CrossRef PubMed.
- L. Molander, M. Ågerstrand and C. Rudén, WikiPharma – A freely available, easily accessible, interactive and comprehensive database for environmental effect data for pharmaceuticals, Regul. Toxicol. Pharmacol., 2009, 55, 367–371 CrossRef CAS PubMed.
- M. Ågerstrand, et al., An academic researcher's guide to increased impact on regulatory assessment of chemicals, Environ. Sci.: Processes Impacts, 2017, 19, 644–655 Search PubMed.
- K. J. Noone, Beware the impact factor, Ambio, 2016, 1–3, DOI:10.1007/s13280-016-0777-6.
- Y. Ohlsson, et al., National Reports with a Review and Synthesis of the Collated Information-Sweden, Final version as of 01.03.2016 of deliverable 2.5 – section on Sweden – of the HORIZON 2020 project INSPIRATION, EC Grant agreement no: 642372, UBA, Dessau-Roßlau, Germany, 2016 Search PubMed.
- European Commission, Strategy for a future Chemicals Policy, White Paper. COM (2001) 88 final, http://www.eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52001DC0088%26qid=1510323012697%26from=DE, 2001.
- D. Butler, Publish in Wikipedia or perish, Nature News, 2008, DOI:10.1038/news.2008.1312.
- C. T. A. Moermond, R. Kase, M. Korkaric and M. Ågerstrand, CRED: Criteria for reporting and evaluating ecotoxicity data, Environ. Toxicol. Chem., 2016, 35, 1297–1309 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7em00422b |
| This journal is © The Royal Society of Chemistry 2017 |
