Mechanisms of distinct activated carbon and biochar amendment effects on petroleum vapour biofiltration in soil†
Abstract
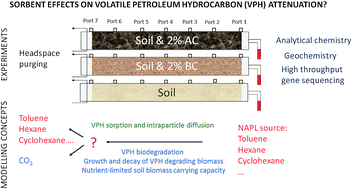
We studied the effects of two percent by weight activated carbon versus biochar amendments in 93 cm long sand columns on the biofiltration of petroleum vapours released by a non-aqueous phase liquid (NAPL) source. Activated carbon greatly enhanced, whereas biochar slightly reduced, the biofiltration of volatile petroleum hydrocarbons (VPHs) over 430 days. Sorbent amendment benefitted the VPH biofiltration by retarding breakthrough during the biodegradation lag phase. Subsequently, sorbent amendment briefly reduced the mineralization of petroleum hydrocarbons by limiting their bioavailability. During the last and longest study period, when conditions became less supportive of microbial growth, because of inorganic nutrient scarcity, the sorbents again improved the pollution attenuation by preventing the degrading microorganisms from being overloaded with VPHs. A 16S rRNA gene based analysis showed sorbent amendment effects on soil microbial communities. Nocardioidaceae benefitted the most from petroleum hydrocarbons in activated carbon amended soil, whereas Pseudomonadacea predominated in unamended soil. Whilst the degrading microorganisms were overloaded with VPHs in the unamended soil, the reduced mobility and bioavailability of VPHs in the activated carbon amended soil led to the emergence of communities with higher specific substrate affinity, which removed bioavailable VPHs effectively at low concentrations. A numerical pollutant fate model reproduced these experimental observations by considering sorption effects on the pollutant migration and bioavailability for growth of VPH degrading biomass, which is limited by a maximum soil biomass carrying capacity. Activated carbon was a much stronger sorbent for VPHs than biochar, which explained the diverging effects of the two sorbents in this study.


 Please wait while we load your content...
Please wait while we load your content...