Evidence of bad recycling practices: BFRs in children's toys and food-contact articles†
Abstract
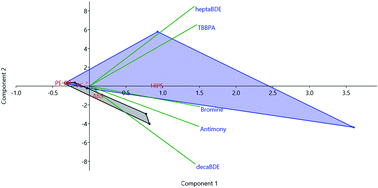
Brominated flame retardants (BFRs) have been used intentionally in a wide range of plastics, but are now found in an even wider range of such materials (including children's toys and food contact articles) as a result of recycling practices that mix BFR-containing waste plastics with “virgin” materials. In this study Br was quantified in toy and food contact samples on the assumption that its concentration can be used as a metric for BFR contamination. Subsequently, compound specific determination of BFRs was performed to evaluate the validity of the aforementioned assumption, crucial to render rapid, inexpensive, in situ Br determination in non-laboratory environments (such as waste handling facilities) a viable option for sorting wastes according to their BFR content. We report semi-quantitative compound specific BFR concentrations to give an overview of the distribution of individual BFRs in the analyzed samples. Finally, we evaluated the correlations between waste electrical and electronic equipment (WEEE) related substances (Ca, Sb and rare earth elements (REEs)) and Br as a proxy for identifying poor sorting practices in different waste streams. 26 samples of toys, food-contact articles and WEEE were analyzed with a suite of different techniques in order to obtain comprehensive information about their elemental and molecular composition. The information obtained from principal component analysis about WEEE-related compounds provides new insights into the influence of sorting practices on the extent of products' contamination and bringing out polymer-related trends in the pollutants' signature. 61% of all samples were Br positive: of these samples, 45% had decaBDE concentrations exceeding the concentration limits for PBDEs and their main constituent polymer was – according to the REE signature of such samples – Acrylonitrile Butadiene Styrene (ABS), uses of which include copying equipment, laptops and computers. The ability to better track chemicals of concern and their trends in products is the main requirement for high-level management and control of material cycles to become non-toxic in the future as proposed in the EU's 7th Environmental Action Plan.


 Please wait while we load your content...
Please wait while we load your content...