Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of ageing on the properties and polycyclic aromatic hydrocarbon composition of biochar†
Gabriel
Sigmund
 a,
Thomas D.
Bucheli
b,
Isabel
Hilber
b,
Vesna
Micić
a,
Melanie
Kah
a,
Thomas D.
Bucheli
b,
Isabel
Hilber
b,
Vesna
Micić
a,
Melanie
Kah
 *a and
Thilo
Hofmann
*a and
Thilo
Hofmann
 *a
*a
aUniversity of Vienna, Department of Environmental Geosciences and Environmental Science Research Network, Althanstrasse 14, UZA2, 1090 Vienna, Austria. E-mail: melanie.kah@univie.ac.at; thilo.hofmann@univie.ac.at; Tel: +43-1-4277-53381 Tel: +43-1-4277-53320
bEnvironmental Analytics, Agroscope Reckenholzstrasse 191, 8046 Zürich, Switzerland
First published on 25th April 2017
Abstract
The influence of ageing on biochar properties has been investigated by comparing three fresh biochars with biochars artificially aged by either H2O2 thermal oxidation or horseradish peroxidase enzymatic oxidation. In addition, a field-aged counterpart for one of the biochars was recovered from an agricultural field site, four years after application. Biochar properties, including surface areas and pore volumes (derived by N2 and CO2 physisorption) and elemental compositions, showed only minor changes following both artificial and field ageing, indicating high biochar stability. Concentrations of the 16 US EPA PAHs were measured in all of the biochars and a contaminant trap was used to investigate the effect of ageing on their bioaccessibility. The concentrations of total and bioaccessible PAHs ranged from 4.4 to 22.6 mg kg−1 and 0.0 to 9.7 mg kg−1, respectively. Concentrations of the 16 US EPA PAHs decreased following field ageing, but the proportion of low molecular weight PAHs increased. The observed changes in PAH composition with field ageing can be explained by uptake from the surrounding soil and intra-biochar transfer processes. To better understand changes in PAH composition with ageing, an additional broad range of alkylated PAHs was also analyzed in selected samples. Our results show that the tested artificial ageing protocols are unable to approximate the changes in PAH composition resulting from field ageing. Nevertheless, total and bioaccessible PAH concentrations decreased for both artificially and field-aged biochars, indicating that biochars release PAHs when they are freshly produced and that the risk of PAH release decreases with ageing.
Environmental impactBiochar is a product of biomass pyrolysis with a range of applications, including agriculture, carbon sequestration and soil/sediment remediation. During the production process PAHs can be formed and their release in the environment upon field application may be of concern. In this study, for the first time, we compare PAH and alkylated PAH composition in fresh biochar, artificially-aged biochar and field-aged biochar. In addition, we applied a contaminant trap method to evaluate PAH bio-accessibility. Total and bioaccessible PAH concentrations decreased for both artificially and field-aged biochars, indicating that biochars release more PAHs when they are freshly produced and that the risk of PAH release decreases with ageing. |
1. Introduction
Biochar, which is a carbon-rich product of biomass pyrolysis, is considered an environmentally friendly carbon sink as well as a valuable soil additive that can enhance soil water-holding capacity and counteract soil acidification.1 Since biochar has a high sorption potential for a large range of contaminants its use has also been proposed for the remediation of soils and sediments.2,3 The properties of biochar are known to change following its application to soil, as a result of ageing processes.1,4 Ageing can, for instance, modify the bulk elemental composition and porosity of biochar,4 as well as the composition and bioaccessibility of intrinsic organic compounds such as polycyclic aromatic hydrocarbons (PAHs). However, the effect of ageing on the composition and bioaccessibility of PAHs that are co-produced with biochar during the biomass pyrolysis has, to our knowledge, hardly been investigated to date.5In recent years a range of methods have been proposed to simulate and accelerate the processes that occur during biochar ageing. This is a challenging task, as field ageing encompasses a range of simultaneous processes that involve physical, chemical and biological alterations over a prolonged period of time. Hale et al.6 proposed protocols to investigate the individual effects that chemical, biological, and physical ageing of biochar have on pyrene sorption. Zhou et al.7 suggested loading biochars with humic acids to represent what could be referred to as fouling, and Cross and Sohi8 proposed thermal oxidation with H2O2 to assess biochar stability. In addition, Flores-Cervantes et al.9 showed that highly stable carbon materials, such as carbon nanotubes, can be partially degraded by enzymatic oxidation with horseradish peroxidase (HRP). This enzymatic ageing approach has not previously been applied to biochar but may provide additional insight into environmental ageing processes.
A comparison of artificially-aged biochar with field-aged biochar is hardly possible, due to the difficulty to separate and quantify biochar after long term incubation in soil. Separation of char particles from soil can involve the use of organic solvents such as sodium-polytungstate for density separation,10 and biochar in soils can be quantified using molecular markers such as benzenecarboxylic acid.11 However, those methods are very laborious and generally involve the use of organic solvents. In this study, we have been able to manually separate floating biochar particles from soil–water suspensions and to quantify the amount of biochar in the soil on the basis of its total organic carbon content (see Section 3.1).
The PAH composition of biochar (by which we mean the relative proportions of different PAHs in biochar) is known to vary depending on their feedstock and production conditions.1 Materials containing PAHs also generally contain alkylated PAHs,12,13 which are more hydrophobic than their unsubstituted counterparts, making them less mobile, more prone to bio-accumulation,14 and hence of relevance in biochar risk assessment. The structural difference between alkylated PAHs and their unsubstituted counterparts may also influence their sorption behavior, i.e., alkylated PAHs may absorb more strongly due to their higher hydrophobicity, but may adsorb less strongly due to their non-planar structure.15 Changes in the PAH and alkylated PAH compositions in biochar with ageing have therefore been expected, but have not previously been investigated in any detail.
In view of these gaps in our knowledge, the aim of this study was to assess the changes in biochar with ageing, with a specific focus on (i) changes in the PAHs content, composition, and bioaccessibility, and (ii) compositional differences of alkylated PAHs between artificially-aged and field-aged biochar. In addition, a simple method is proposed for the quantification of biochar in field samples amended at relatively high rates.
2. Materials and methods
2.1 Sample preparation and ageing
Two standard biochars produced from Miscanthus at 550 °C and 700 °C (MSP550 and MSP700) were purchased from the UK Biochar Research Center (Edinburgh, United Kingdom). A biochar (Romchar, <2 cm) produced from softwood at 550 °C had previously been applied (in March 2011) to an agricultural field site in Traismauer, Lower Austria, at a relatively high rate of 72 t ha−1.16 Samples of the fresh material have since then been stored in a dark, dry room and soil samples were subsequently collected from the agricultural field site in October 2015. The field-aged Romchar (referred to herein as “Romchar-Field”) particles were lighter than the soil particles and could therefore be separated from a soil–water suspension. Soil samples (10–20 g) were suspended in 200 mL ultrapure water (Millipore, Elix 5-Milli-Q Gradient A10) and shaken. The floating biochar particles in the soil suspensions were then separated manually, the water discarded, and the process repeated several times with fresh water. Finally, the separated biochar was dried at 105 °C for 2 h.All biochars were crushed with a mortar and pestle and sieved to <250 μm. Subsamples of the three fresh biochars (MSP550, MSP700, and Romchar) were chemically aged by a thermal oxidation treatment with 0.01 mol L−1 H2O2 at 80 °C for 48 h.8 Other subsamples were aged by enzymatic oxidation with horseradish peroxidase, by adapting a method presented by Allen et al.17 A biochar suspension (1 g L−1) was prepared in a background solution consisting of 0.01 mol L−1 phosphate-buffered saline adjusted to a pH of 6, with 600 enzyme units per L of horse radish peroxidase (Sigma Aldrich, PCode: 1002025248), adapting a method by Flores-Cervantes et al.9 Following incubation for 24 h the enzyme was activated with 500 μmol L−1 H2O2 and the biochar suspensions placed on a magnetic stirrer (300 rpm) for 10 d. The slurries from both chemical and enzymatic ageing were then filtered and the >0.1 μm fraction dried at 105 °C for 2 h.
2.2 Bulk characterization
The total organic carbon content (TOC) of the biochars and soils was determined using a carbon analyzer equipped with a solid-state infrared detector (LECO RC-612). The ash content was determined by weighing samples before and after heating at 750 °C for 6 h.18 The total C, H, N and S contents were determined using an elemental analyzer (Elementar VarioMacro); the O content was calculated by mass balance: O% = 100 − (C + H + N + S + ash). For fresh biochars, the stable carbon fraction was calculated on the basis of the carbon content and changes in weight following H2O2 ageing.8 Specific surface areas, pore volumes, and pore-size distributions were derived from N2 and CO2 physisorption isotherms following degassing overnight at 105 °C (Quantachrome Nova 2000 analyzer, see Sigmund et al.19 for details). Scanning electron microscope images were obtained with an Inspect™ S50 Scanning Electron Microscope operating under high vacuum (10 kV) and using an Everhart–Thornley Detector.2.3 PAH composition and desorption-resistant fractions determined with contaminant traps
Glass vessels containing a bottom layer of silicon and activated carbon were loaded with either 100 mg of biochar or 5 g of soil, and with 25 mL of 140 g L−1 cyclodextrin solution. Two replicates were prepared for each of the samples. Additional vessels with cyclodextrin solution but no activated carbon layer were prepared as controls.20 All samples were then incubated in the dark at room temperature (22 °C) for 30 d. Thereafter the samples were filtered and the >0.2 μm fraction placed into a Soxhlet extraction thimble with approximately 50 mg of NaSO4 and a spatula tip of copper. After adding deuterated internal standards (d8-naphthalene, d8-acenaphthylene, d10-acenaphthene, d10-fluorene, d10-phenanthrene, d10-anthracene, d10-fluoranthene, d10-pyrene, d12-benzo[a]anthracene, d12-chrysene, d12-benzo[b]fluoranthene, d12-benzo[k]fluoranthene, d12-benzo[a]pyrene, d12-indeno[1,2,3-cd]pyrene, d14-dibenz[a,h]anthracene, and d12-benzo[ghi]perylene) the extraction thimble was placed in the Soxhlet apparatus and extracted with 130 mL of toluene for 36 h. The extracts were subsequently reduced down to 1 mL using a Syncore parallel evaporator (Büchi, Flawil, Switzerland), heating to 70 °C until the target volume was reached.20 The concentrated extracts were then cleaned up using a dimethylformamide-based method and finally, the 16 US EPA PAHs were measured using gas chromatography-mass spectrometry. The bioaccessible PAHs were quantified as the difference between total PAHs (measured in the cyclodextrin control) and desorption-resistant PAHs (measured after incubation in the contaminant trap).20 To test for losses in the filtration process a liquid/liquid extraction of five filtrates (all fresh biochars) was performed with hexane, showing that losses of the 16 US EPA PAHs were <0.6 mg kg−1.In addition, alkylated PAHs were analyzed using a gas chromatography-mass spectrometry method developed by Laumann et al.21 for a selected group of extracts. The limit of quantification (LoQ) was <0.01 mg kg−1 for all PAHs.
2.4 Data analysis
Data were analyzed using SigmaPlot 12.5 and R x64 3.3.0 software. All data values below the LoQ were replaced with random numbers between the specific LoQ and one tenth of its value. In the statistical analysis, p values <0.05 were considered significant.3. Results & discussion
3.1 Field-aged biochar
In March 2011 72 t ha−1 of Romchar was incorporated in the top 10 cm of a loamy clay soil, corresponding to approximately 4.80% (dry weight) of biochar. Soil (0–15 cm deep) was recovered from the site in October 2015 and field-aged biochar particles separated from the soil (see Section 2.1). Bulk chemical-physical characterization of Romchar-Field compared to fresh Romchar exhibited only minor physical and chemical alteration following the field ageing (see Table S1 in the ESI†), indicating (i) a reasonable separation of the biochar from the soil, and (ii) that degradation/dissipation of the biochar was only a minor process.The TOC values obtained for amended and unamended soil samples indicate that 2.58 ± 0.01% of the original 4.80% of biochar remained in the amended soil four years after application (for further details see Fig. S1 in the ESI†), which corresponds to a biochar mass loss of approximately 46%. The validity of our quantification of biochar mass was confirmed by comparing the measured content of the 16 US EPA PAHs in the unamended soil, the amended soil, and the Romchar-Field. The concentrations of these PAHs measured in the biochar-amended soil (corrected for the biochar content and the PAH concentrations in the unamended soil) corresponded well to those in Romchar-Field (6.40 ± 1.14 mg kg−1 and 5.83 ± 0.22 mg kg−1, respectively), indicating a reasonable estimation of the biochar mass based on TOC. It should be noted that the field site used in this study was treated with a relatively large quantity of biochar (72 t ha−1). A trend towards lower application rates has been observed over recent years,22 and the quantification of biochar on the basis of TOC measurements may not be possible if application rates are very low. The mixing with unamended soil, and transport of biochar to the subsurface and/or off site as a result of ploughing (0–20 cm deep, at least once a year) was probably the main reason for the reduced amount of biochar in the soil. A predominance of transport off site as a dissipation process would be in good agreement with published literature on biochar dissipation1,23 and the findings of a recently published review on biochar stability.24
In order to assess how well artificial ageing protocols (based on chemical and enzymatic oxidation) are able to reproduce the results of natural ageing, fresh Romchar was artificially aged and the results compared with those from Romchar-Field. Bulk chemical characterization indicated only minor changes following artificial ageing but a reduction in the measured specific surface areas and pore volumes. In contrast, both the specific surface areas and the pore volumes increased slightly following field ageing (see Table S1 in the ESI† for bulk properties).
The pore-size distributions obtained from N2 and CO2 physisorption (Fig. 1) indicated that, following field ageing, the contribution of smaller pores to the overall pore volume increased, possibly due to the break-down and clogging of macro-structures in the field. In contrast, the contributions of smaller pores to the specific surface area, and the pore volume all decreased following artificial ageing. Possibly due to destruction and rinsing out of the smallest biochar fraction during the ageing process, as smaller particles may have been preferentially oxidized due to higher accessible (macro)surface areas. This hypothesis is supported by the SEM images of the Romchar biochars shown in Fig. 2, in which pores tend to be more visible in artificially-aged biochar than in field-aged biochar, where macrostructures are broken down and macropores filled in.
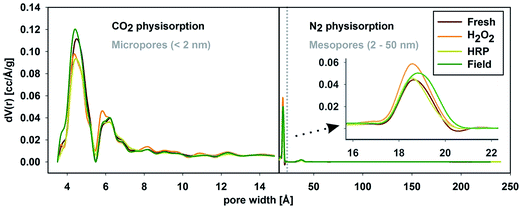 | ||
| Fig. 1 Pore-volume-weighted pore-size distributions for fresh Romchar and for H2O2-, HRP- or field-aged Romchar derived from CO2 and N2 physisorption. | ||
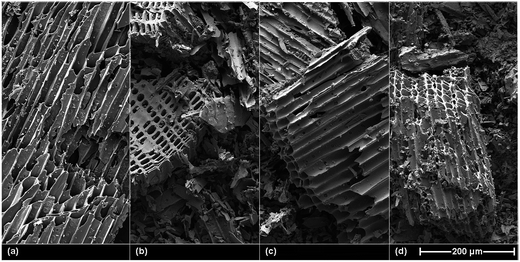 | ||
| Fig. 2 Scanning electron microscopy (SEM) images of fresh (a), H2O2-aged (b), HRP-aged (c), and field-aged (d) Romchar. | ||
To investigate whether the observed trends resulting from artificial ageing of Romchar could be generalized to other biochars, two additional biochars (MSP550 and MSP700) were also tested; their bulk characterisation results can be found in Table S1 and Fig. S2 in the ESI.† Changes in the bulk characteristics were generally small in all of the biochars tested, indicating a high level of stability. The stable carbon in the three fresh biochars ranged from 92.2 to 97.8% of the original carbon content, based on the method proposed by Cross and Sohi8 (see Table S1 in the ESI†).
3.2 PAH composition
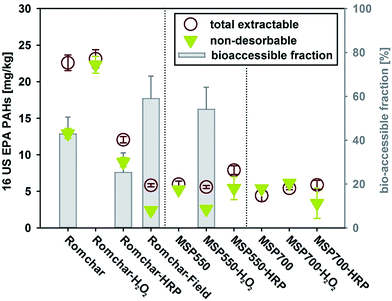
The MSP550 and MSP700 biochars contained ≤6 mg kg−1 of the 16 US EPA PAHs whereas Romchar contained >22 mg kg−1, thereby exceeding the European biochar certificate (EBC) threshold for basic grade biochar of 12 mg kg.25 The H2O2 ageing had no effect on the total PAH content of Romchar, whereas both field ageing and enzymatic ageing significantly reduced its total PAH content (see Table S2† and Fig. 4). Neither H2O2 ageing nor enzymatic ageing had any significant impact on the total PAH content in MSP550 and MSP700 biochars, indicating that PAHs may be less susceptible to degradation in MSP500 and MSP700 than in Romchar.Artificial ageing tended to reduce the relative content of low molecular weight (LMW) PAHs (with ≤three aromatic rings). Due to their lower lipophilicity and higher solubility, LMW PAHs are expected to be more readily desorbed from biochar than high molecular weight (HMW) PAHs (with ≥four aromatic rings). A greater release of LMW PAHs during the artificial ageing protocol (biochar in suspension) drove changes in relative PAH composition for H2O2-, and HRP ageing (see Fig. 3 for relative PAH compositions).
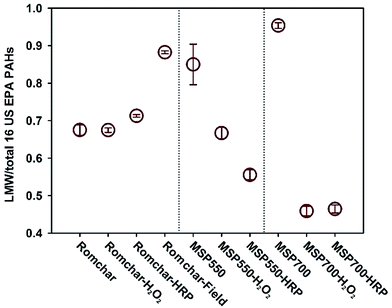 | ||
| Fig. 3 Changes in the proportion of low molecular weight (LMW) PAHs for three types of biochar, depending on the type of ageing. | ||
In contrast to artificial ageing, field ageing led to a reduction in the total PAH concentrations but an increase in the proportion of LMW PAHs in Romchar-Field. These changes can be explained by (i) uptake of LMW PAHs from the surrounding soil, and/or (ii) a higher intra-biochar liquid–liquid phase transfer of smaller molecules into micropores. Smaller LMW PAH molecules are less lipophilic and experience less steric hindrance than HMW PAHs. Hence, LMW-PAHs are more mobile and able to access micropores that are not accessible to HMW PAHs.26,27 The PAH concentrations in the unamended soil and Romchar-Field were similar after normalization to TOC content and hence PAHs, especially mobile LMW PAHs, may have migrated from the soil into the biochar. This is consistent with the accumulation of environmental PAHs in coals within river sediments that has previously been observed by Yang et al.28 Sorption of LMW PAHs due to intra-biochar transfer may also be a plausible explanation for the observed changes in PAH composition, as (i) the measured pore-size distribution of Romchar-Field (see Fig. 1) revealed the presence of pores within the size range in which the hypothetical molecular sieving effect can be expected, and (ii) HMW PAHs appeared to desorb more readily from Romchar-Field than LMW PAHs in the contaminant trap experiments (see next Section).
Overall, both uptake from the surrounding soil and intra-biochar transfer of LMW PAHs may contribute to the increased proportion of LMW PAHs following field ageing. Assessment of their relative contributions to changes in PAH composition will require additional investigations, which were beyond the scope of this study.
3.3 Bioaccessible PAH fraction
Mayer et al.29 found good agreement between contaminant trap determinations of PAHs desorbable from soil and PAH biodegradation measurements, indicating that only the desorbable PAHs were bioaccessible. Consistently, in this study, the bioaccessible PAHs were quantified as the difference between total PAHs and contaminant trap desorption-resistant PAHs (see Section 2.3 for further details).Romchar biochars had the largest concentration of bioaccessible PAHs (>9 mg kg−1 of the 16 US EPA PAHs for fresh Romchar, Fig. 4) and the proportion of bioaccessible PAHs was reduced by both artificial ageing methods. The total PAHs concentration was reduced by field ageing, indicating that PAHs were released in the field. However, the proportion of bioaccessible PAHs was not significantly altered by field ageing (42.8 ± 7.8 and 58.1 ± 10.2 for fresh and field aged Romchar, respectively), indicating that fresh biochar presents the greatest risk with regard to PAH release. The bioaccessible PAH fractions for MSP550 and MSP700 were generally negligible for the artificially-aged samples and associated with high levels of uncertainty, possibly because of their generally low total PAH concentrations.
The proportion of LMW PAHs in Romchar and artificially-aged Romchar (approx. 0.70 LMW/16 US EPA PAHs for all) did not change significantly as a result of incubation in the contaminant trap, but increased for Romchar-Field (from 0.88 to 0.92 LMW/16 US EPA PAHs). It is interesting to note that H2O2 ageing reduced the bioaccessibility of PAHs from Romchar but increased the bioaccessibility of PAHs from MSP550. The different effects that H2O2 ageing has on the different biochars could be due to differences in their structures, as well as other factors such as surface chemistry, however the detailed processes involved remain unknown. As with the total PAHs concentrations, the H2O2 ageing of Romchar was not able to reproduce the changes in PAH bioaccessibility that resulted from field ageing. The HRP ageing was better able to approximate changes in both the total concentration of PAHs and their bioaccessible fraction in Romchar-Field, and indicated that PAHs in MSP550 and MSP700 are not expected to become more accessible as a result of ageing.
Overall, both the 16 US EPA PAHs concentration in Romchar and the proportion of bioaccessible PAHs were relatively high, whereas those for the two Miscanthus-based biochars (MSP550 and MSP700) were generally negligible (within error). Our results indicate that biochars that do not meet biochar quality standards25,30 can present a risk of PAHs release when applied at high dosages, whereas biochars that have been produced under well-controlled conditions and meet those standards are unlikely to present such a risk. This is in good agreement with recently published findings by Hilber et al.31
3.4 Alkylated PAHs
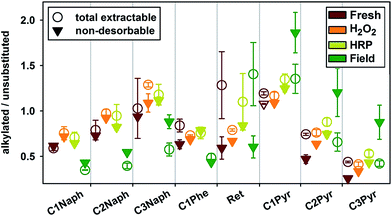
The total concentrations of PAHs and alkylated PAHs followed similar trends; the subsequent discussion will therefore focus on changes in their relative proportions. Artificial ageing generally increased the proportion of alkylated compounds compared to their unsubstituted counterparts, especially for naphthalene (see Fig. 5). This can possibly be explained by the lower KOW of the unsubstituted compounds, making them more easily desorbable from the biochars during the artificial ageing process (e.g. the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW is 3.41 for naphthalene and 3.88 for 1-methylnaphtalene32). In contrast, field ageing generally reduced the proportion of alkylated compounds, possibly because the smaller unsubstituted compounds were more easily transferred into biochar micropores (see Section 3.2) while the larger alkylated compounds remained more easily desorbable and accessible for degradation.
KOW is 3.41 for naphthalene and 3.88 for 1-methylnaphtalene32). In contrast, field ageing generally reduced the proportion of alkylated compounds, possibly because the smaller unsubstituted compounds were more easily transferred into biochar micropores (see Section 3.2) while the larger alkylated compounds remained more easily desorbable and accessible for degradation.
Sorption of unsubstituted PAHs to biochar, including the stacking of PAHs on graphene-like aromatic biochar surfaces, is often discussed in terms of π–π interactions which are favored by the planarity of PAH molecules.33 The alkylation of PAHs makes the molecules non-planar, while at the same time increasing their hydrophobicity. Alkylation may therefore reduce the relative contribution of π–π interactions to the overall sorption and increase the role of hydrophobicity-driven interactions.15 The reduction in the proportion of alkylated compounds following field ageing may therefore be explained by increased absorption of more alkylated PAHs into amorphous organic matter domains of biochar. This hypothesis is in good agreement with results from the contaminant trap experiments, which indicated a larger proportion of bioaccessible alkylated compounds in fresh Romchar than of the respective unsubstituted compounds (see Fig. 5).
The proportion of alkylated compounds in field-aged biochar increased after the contaminant trap experiments. This can be explained by (i) the higher microbial degradation of accessible alkylated PAHs in the field due to proportionally greater absorption into amorphous organic matter than to unsubstituted PAHs, and (ii) pore deformation, which has previously been suggested to play a key role in the release of naphthalene from a reference coal34 and may also depend on the molecular structure.
In contrast to the other measured alkylated compounds, the concentrations of the biomarker retene decreased for all samples following removal of the bioaccessible fraction in the contaminant trap experiments (see Fig. 5). Retene is commonly found in natural environments and is the least planar compound measured; it can originate from a variety of sources, including wood burning, diagenetic processes, traffic emissions, and waste incineration.35 A background concentration of approximately 0.06 mg kg−1 of retene was measured in the unamended soil from the field site used for these investigations. Retene was also the most abundant alkylated compound measured in the fresh Romchar. The distinctive behavior of retene in field-aged biochar can be explained by (i) the generally high initial retene concentration in the biochar, (ii) retene being accumulated on the biochar from the surrounding soil, and (iii) the greater contribution of absorption to the overall sorption compared to more planar compounds.
Changes in the proportions of alkylated compounds as a result of field ageing could not be simulated by artificial ageing protocols and were not always consistent between compound groups. The alkylated PAH compositions following field ageing are likely to have been affected by a variety of interconnected interactions including intra-biochar transfers, desorption, and microbial degradation. There is currently no detailed understanding of the effects that individual interactions have on each other and this should be the focus of future investigations.
4. Conclusion
Artificial ageing may be useful for investigating changes in specific biochar properties as a result of field ageing, but the representativeness of the applied ageing methods needs to be critically evaluated on a case by case basis. For instance, the reduction in total PAHs content produced by enzymatic ageing with HRP more closely resembled the results of field ageing than did the results of H2O2 ageing, but none of the tested artificial ageing methods were able to fully reproduce the changes in PAH composition produced by field ageing. In contrast to Romchar, enzymatic ageing had no effect on the other biochars (MSP550 and MSP700), which is consistent with the proportions of bioaccessible PAHs (i.e. up to 50% for Romchar and negligible for MSP550 and MSP700). These results indicate that well-produced biochars that meet European Biochar Certificate (EBC) and International Biochar Initiative (IBI) quality thresholds for total PAH concentrations are unlikely to present a risk with regard to PAH release following field application. Applications of biochar with low initial PAHs concentrations can therefore be assumed to be safe. Biochars that do not meet those quality standards (such as the Romchar used in this study) require more critical evaluation, as they may well present a risk, particularly at high application rates.The artificial ageing protocols tested (i.e., H2O2 ageing and HRP ageing) were unable to approximate the changes in PAH composition that resulted from field ageing. For instance, the proportion of LMW PAHs generally decreased as a result of artificial ageing but increased following field ageing. The observed discrepancies are likely to be due to differences in process kinetics, including the intra-biochar transfer of PAHs and the sorption of LMW PAHs from the surrounding environment. Due to the lack of data in literature, further investigations are necessary especially on the hypothesized differences in intra-biochar transfer of PAHs. Some changes in the PAHs and alkylated PAH compositions as a result of ageing can be explained, but interpreting changes in alkylated PAH compositions generally remains difficult. Detailed investigations into (i) intra-biochar transfer of PAHs and alkylated PAHs, (ii) the kinetics of desorption hysteresis of methylated compounds compared to parent compounds, and (iii) factors driving microbial degradation of PAHs co-produced with biochar during pyrolysis, will therefore be required to further our understanding of the fate of biochar-intrinsic PAHs and alkylated PAHs in natural environments.
Acknowledgements
This study was funded by the Austrian Federal Ministry of Agriculture, Forestry, Environment and Water Management (BMLFUW), management by Kommunalkredit Public Consulting GmbH (grant number B420004). Additional funding for a short term scientific mission of Gabriel Sigmund was provided by the European COST framework (Action TD1107). We are also grateful to Gerhard Soja and Rebecca Hood-Nowotny (Austrian Institute of Technology) for providing the Romchar and access to the field site to sample field-aged biochar.References
- J. Lehmann and S. Joseph, Biochar for Environmental Management – Science, Technology and Implementation, Routledge, London Sterling VA, 2nd edn, 2015 Search PubMed.
- M. Ahmad, A. U. Rajapaksha, J. E. Lim, M. Zhang, N. Bolan, D. Mohan, M. Vithanage, S. S. Lee and Y. S. Ok, Chemosphere, 2014, 99, 19–33 CrossRef CAS PubMed.
- I. Hilber, A. C. Bastos, S. Loureiro, G. Soja, A. Marsz, G. Cornelissen and T. D. Bucheli, J. Environ. Eng. Landsc., 2017 DOI:10.3846/16486897.2016.1254089.
- G. Sorrenti, C. a. Masiello, B. Dugan and M. Toselli, Sci. Total Environ., 2016, 563–564, 237–246 CrossRef CAS PubMed.
- S. E. Hale, J. Lehmann, D. Rutherford, A. R. Zimmerman, R. T. Bachmann, V. Shitumbanuma, A. O'Toole, K. L. Sundqvist, H. P. H. Arp, G. Cornelissen and A. O'Toole, Environ. Sci. Technol., 2012, 46, 2830–2838 CrossRef CAS PubMed.
- S. E. Hale, K. Hanley, J. Lehmann, A. Zimmerman and G. Cornelissen, Environ. Sci. Technol., 2011, 45, 10445–10453 CrossRef CAS PubMed.
- Z. Zhou, H. Sun and W. Zhang, Environ. Pollut., 2010, 158, 1916–1921 CrossRef CAS PubMed.
- A. Cross and S. P. Sohi, GCB Bioenergy, 2013, 5, 215–220 CrossRef CAS.
- D. X. Flores-Cervantes, H. M. Maes, A. Schäffer, J. Hollender and H.-P. E. Kohler, Environ. Sci. Technol., 2014, 48, 4826–4834 CrossRef CAS PubMed.
- Y. Yang, B. Ligouis, C. Pies, C. Achten and T. Hofmann, Chemosphere, 2008, 71, 2158–2167 CrossRef CAS PubMed.
- B. Glaser, L. Haumaier, G. Guggenberger and W. Zech, Org. Geochem., 1998, 29, 811–819 CrossRef CAS.
- M. Keiluweit, M. Kleber, M. a. Sparrow, B. R. T. Simoneit and F. G. Prahl, Environ. Sci. Technol., 2012, 46, 9333–9341 CrossRef CAS PubMed.
- M. B. Yunker, R. W. Macdonald, R. Vingarzan, R. H. Mitchell, D. Goyette and S. Sylvestre, Org. Geochem., 2002, 33, 489–515 CrossRef CAS.
- J. T. Andersson and C. Achten, Polycyclic Aromat. Compd., 2015, 35, 330–354 CrossRef CAS PubMed.
- M. T. O. Jonker and F. Smedes, Environ. Sci. Technol., 2000, 34, 1620–1626 CrossRef CAS.
- J. Karer, B. Wimmer, F. Zehetner, S. Kloss and G. Soja, Agric. Food Sci., 2013, 22, 390–403 Search PubMed.
- B. L. Allen, G. P. Kotchey, Y. Chen, N. V. K. Yanamala, J. Klein-Seetharaman, V. E. Kagan and A. Star, J. Am. Chem. Soc., 2009, 131, 17194–17205 CrossRef CAS PubMed.
- ASTM D 1762-84, ASTM Int., 2011, 84, 1–2.
- G. Sigmund, T. Hüffer, T. Hofmann and M. Kah, Sci. Total Environ., 2017, 580, 770–775 CrossRef CAS PubMed.
- P. Mayer, I. Hilber, V. Gouliarmou, S. E. Hale, G. Cornelissen and T. D. Bucheli, Environ. Sci. Technol., 2016, 50, 1941–1948 CrossRef CAS PubMed.
- S. Laumann, V. Micić, M. A. Kruge, C. Achten, R. F. Sachsenhofer, J. Schwarzbauer and T. Hofmann, Environ. Pollut., 2011, 159, 2690–2697 CrossRef CAS PubMed.
- H. P. Schmidt, B. H. Pandit, V. Martinsen, G. Cornelissen, P. Conte and C. Kammann, Agriculture, 2015, 5, 723–741 CrossRef.
- O. Masek, P. Brownsort, A. Cross and S. Sohi, Fuel, 2013, 103, 151–155 CrossRef CAS.
- J. Wang, Z. Xiong and Y. Kuzyakov, GCB Bioenergy, 2016, 8, 512–523 CrossRef CAS.
- EBC, 2015, 1–22.
- C. Lattao, X. Cao, J. Mao, K. Schmidt-Rohr and J. J. Pignatello, Environ. Sci. Technol., 2014, 48, 4790–4798 CrossRef CAS PubMed.
- M. Kah, H. Sun, G. Sigmund, T. Hüffer and T. Hofmann, Bioresour. Technol., 2016, 214, 225–233 CrossRef CAS PubMed.
- Y. Yang, B. Ligouis, C. Pies, P. Grathwohl and T. Hofmann, Environ. Pollut., 2008, 151, 121–129 CrossRef CAS PubMed.
- P. Mayer, J. L. Olsen, V. Gouliarmou, M. Hasinger, R. Kendler and A. P. Loibner, Environ. Sci. Technol., 2011, 45, 2932–2937 CrossRef CAS PubMed.
- IBI, 2015, 1–47.
- I. Hilber, P. Mayer, V. Gouliarmou, S. E. Hale, G. Cornelissen, H.-P. Schmidt and T. D. Bucheli, Chemosphere, 2017, 174, 700–707 CrossRef CAS PubMed.
- ARChem, 2016.
- M. Keiluweit and M. Kleber, Environ. Sci. Technol., 2009, 43, 3421–3429 CrossRef CAS PubMed.
- M. Sander and J. Pignatello, Environ. Sci. Technol., 2005, 39, 7476–7484 CrossRef CAS PubMed.
- R. C. Brändli, T. D. Bucheli, S. Ammann, A. Desaules, A. Keller, F. Blum and W. A. Stahel, J. Environ. Monit., 2008, 10, 1278–1286 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7em00116a |
| This journal is © The Royal Society of Chemistry 2017 |