Impact of environmental conditions on bacterial photoreactivation in wastewater effluents†
Shirin
Shafaei
a,
Nikolaus
Klamerth
ab,
Yanyan
Zhang
a,
Kerry
McPhedran
ac,
James R.
Bolton
a and
Mohamed
Gamal El-Din
*a
a7-285 Donadeo Innovation Centre for Engineering, Environmental Engineering Program, Department of Civil and Environmental Engineering, University of Alberta, Edmonton, Alberta T6G 1H9, Canada. E-mail: mgamalel-din@ualberta.ca; Tel: +1 780 492 5124
bDepartment of Hydrogeology, Freiberg University of Mining and Technology, Freiberg, Saxony, Germany
cDepartment of Civil and Geological Engineering, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
First published on 14th December 2016
Abstract
Photoreactivation is a process where ultraviolet (UV)-induced damage to the DNA of microorganisms can be reversed by exposure to near UV and visible light. To date, most photoreactivation experiments have been carried out under laboratory conditions using standard microorganisms that do not reflect the natural conditions of municipal wastewater effluents. Photoreactivation could increase the concentration of pathogens released into natural systems, leading to negative impacts on fish, shellfish, and clams. In addition, pathogen release can increase health risks of downstream activities, such as swimming. This study focused on the photoreactivation of total coliforms in municipal wastewater effluents under natural sunlight conditions. The concept of ‘effective reactivation fluence’ (ERF) is used to evaluate and normalize the results from various light sources for a direct comparison. ERF values higher than 30 J cm−2, in conjunction with lowered nutrient concentrations (dilution of effluents with river water), decreased the photoreactivation of total coliforms. In contrast, higher temperatures (up to 25 °C) and blocking the UV-B portion of natural sunlight using a polyethylene terephthalate (PET) bottle increased their photoreactivation. The results of this research will provide guidance to wastewater plant operators on the potential need to minimize the level of photoreactivation in effluents before the effluents were released into receiving water bodies.
Environmental impactUltraviolet (UV) treatment has been used increasingly to replace chlorination as an alternative disinfection method. However, photoreactivation needs to be considered especially when the discharged wastewater effluents are exposed to sunlight in surface waters immediately after the UV treatment. Photoreactivation could increase the number of bacteria in receiving water, which poses a risk to fish and other organisms. This study is the first to investigate the significant factors related to photoreactivation under natural sunlight and real outdoor conditions for an actual wastewater effluent using an “effective reactivation fluence” concept. The findings of this study are environmentally important and could be beneficial for establishing standards for safe discharge of treated municipal wastewater effluents to receiving waters. |
1. Introduction
Ultraviolet (UV) treatment has been used increasingly to replace chlorination as an alternative disinfection method in many municipal drinking water and wastewater treatment plants. UV has several advantages over chlorination including a higher disinfection efficiency for most viruses, bacteria and protozoa, no identified toxic disinfection by-products (DBPs) and ease of operation.1 Exposure of pathogens to UV light, emitted by either low- or medium-pressure mercury vapor lamps at a sufficient inactivation fluence (IF) (i.e., UV dose typically measured in mJ cm−2), results in pathogen inactivation arising from damage to the microorganism nucleic acids (DNA/RNA) and proteins through various mechanisms (e.g., formation of thymine–thymine dimers).2,3 This inactivation prevents DNA replication and transcription that stops pathogen reproduction.4–6 However, microorganisms possess the ability to repair the UV-induced DNA damage by both light-dependent (i.e., photoreactivation) and light-independent (i.e., dark reactivation) mechanisms.1,4–10In many waterborne bacterial species enzyme-mediated photoreactivation uses light in the 310–480 nm wavelength range to repair damaged DNA through the enzyme photolyase.1,5,8 This potential for photoreactivation is a disadvantage of the UV disinfection process, as exposure of treated waters to sunlight after UV treatment can lead to increased microbial concentrations in effluents.1,7 Overall, photoreactivation does not play a significant role in drinking water disinfection due to the limited light exposure in the distribution system. In contrast, it has important implications for disinfection in municipal wastewater treatment plants (WWTPs), where the discharged treated wastewater effluents are exposed to sunlight in surface waters after the UV treatment. This photoreactivation leads to increased pathogen concentrations such as fecal coliforms that may negatively impact fish, shellfish, clams, among other organisms, in receiving waters.11,12 In addition, the pathogens also pose a human health risk for downstream activities including recreational activities (e.g., swimming). Thus, to protect receiving waters and to reduce potential human health risks, it is necessary to investigate the impacts of bacterial repair after UV treatment in WWTP effluents. The combination of inactivation from UV treatment and the reactivation effects of sunlight should be considered simultaneously to monitor their individual impacts on the photoreactivation process.
The reported effective wavelengths for photoreactivation are from 310 and 480 nm; however, there remains a lack of consensus regarding the exact inactivation and photoreactivation wavelengths.13 Herndl et al.14 showed that bacterial activity declined after exposure to solar UV-B (280–315 nm) radiation. Sinton et al.15 found that the effect of the UV-B (280–315 nm) portion of sunlight on the inactivation of E. coli was twice that of UV-A (315–400 nm). Thus, according to these research studies, it would be important to examine the impact of the wavelength on bacterial photoreactivation by blocking the UV-B portion of sunlight. In addition to wavelength, temperature is another important factor influencing photoreactivation. Previous research has shown that higher temperatures (25–35 °C) significantly increase bacterial photoreactivation under both indoor and outdoor conditions.16–18
One of the principal methods to control bacterial photoreactivation is the application of sufficiently high IF.1,17,19–24 For example, an IF greater than 15 mJ cm−2 has been shown to result in limited photoreactivation of total coliforms, fecal coliforms, and E. coli as compared to lower IF when photoreactivation fluence is kept constant.1,20
To date, the focus of previous UV treatment studies has been on individual species (e.g., standard bacteria) and laboratory-based experiments; however, there is a considerable lack of knowledge regarding the response of complex natural communities of microorganisms to UV treatment.6 Moreover, the effects of nutrients (amongst other compounds) in receiving waters should be considered when investigating bacterial photoreactivation.24
To normalize the results of photoreactivation experiments based on the light source, the concept of ‘Effective Reactivation Fluence’ (ERF) is considered. This concept was first introduced by Bohrerova et al.8 to evaluate photoreactivation results. The ERF is the integral of the spectral fluence rate weighted by the reactivation action (i.e., the relative reactivation effect at a given wavelength), multiplied by the exposure time in seconds.
The main objective of this study was to investigate the significant factors related to photoreactivation under natural sunlight and real outdoor conditions for an actual WWTP effluent using the ERF concept. The effects of ERF, temperature, the UV-B portion of sunlight, and the addition of river water on the bacterial photoreactivation (BP) of the municipal wastewater effluent were also investigated. The findings of this study are environmentally important because the majority of the municipal WWTP effluents are delivered into rivers or lake waters that are exposed to sunlight and have the potential of photoreactivation which increases the number of bacteria, posing a risk to fish and other organisms.
2. Materials and methods
2.1. Sampling analysis and bacterial count
Wastewater samples were collected from the influent and effluent of the UV disinfection unit at the Gold Bar municipal WWTP (GBWWTP), Edmonton, AB, Canada and stored in high density polyethylene (HDPE) plastic containers at 4 °C for up to 1 hour. River water samples were collected by using a HDPE plastic bucket from the North Saskatchewan River upstream of the GBWWTP and stored in the same manner. Water parameters, such as pH, color, turbidity, alkalinity, bicarbonate, dissolved organic carbon (DOC), phosphorus and ammonia, were measured for effluent and river water samples according to standard methods.25 The DOC was measured with a TOC analyzer (Apollo 9000 TOC Combustion Analyzer, FOLIO Instruments Inc.) and the samples were filtered using a pre-rinsed cellulose ester filter (pore size of 0.45 μm, Millipore, USA) prior to measurement.A collimated beam apparatus equipped with a 1 kW medium pressure (MP) lamp (Calgon Carbon Corp., Pittsburgh, PA, USA Model No. ps 1-1-120) was used for bacterial disinfection prior to the photoreactivation experiments according to the standard protocol described elsewhere.26 A low pressure (LP) UV lamp (Model No. G12T6L, Atlantic Ultraviolet Corp., Haupauge, NY) was used to compare bacterial inactivation with MP UV lamps. Due to the high irradiance of the MP lamp, which reduced the exposure time for a 5 mJ cm−2 inactivation fluence to less than 30 s even at the longest distance (120 cm) from the lamp to the sample, a steel mesh filter with a mesh size of 0.43 mm was placed in the light path of the MP UV lamp to reduce irradiance and increase the exposure time. The incident irradiance on the sample surface was measured with a calibrated radiometer (International Light Inc. Model IL 1400A) along with a detector (International Light Inc. Model 18 SED240). The irradiance was fixed at 0.14 and 0.24 mW cm−2 (corrected by the sensor factor) throughout the experiment for the MP and LP UV lamps, respectively. The absolute irradiance of sunlight was measured with a spectroradiometer (JAZ-A, Ocean Optics Inc.) with the software program SpectraSuite.
Bacterial counts were recorded using the heterotrophic plate count standard method.25 After light exposure, the samples were diluted [10, 100, and 1000 times], and filtered through a cellulose ester membrane (0.45 μm, Millipore, USA), and the membrane filter was incubated at 37 °C for 24 h on MF-Endo agar to culture total coliforms (used as representative bacteria for all experiments).
2.2. Experimental setup for photoreactivation: the effect of fluence, temperature, wavelength, and river water
Photoreactivation experiments under natural sunlight (outdoors) were carried out in Pyrex® dishes covered with Saran Wrap® to avoid sample evaporation. Controls (dark control) were covered with black plastic. Fluence and temperature effects on BP were investigated using samples from the effluent of the UV disinfection unit at the GBWWTP after applying 23 mJ cm−2 IF as determined by the target wastewater plant. Adjustment of the reactivation fluence for the investigation of fluence effects on BP was achieved by placing three stainless steel mesh filters (mesh sizes of 0.63, 0.42, and 0.25 mm), (Sigma-Aldrich) individually or in combination on top of the dishes.The temperature-effect experiments were conducted using a water bath at temperatures of 5, 10, 15, 20 and 25 °C. The samples were covered with two mesh filters (0.25 mm) to reduce the ERF values.
Wavelength and river water effects on BP were investigated by applying 10 mJ cm−2 IF on the samples collected from the influent of the disinfection UV unit at the GBWWTP. A 10 mJ cm−2 IF was preferred to 23 mJ cm−2 IF to achieve enough percent photoreactivation for investigation. The wavelength effect experiments were conducted in three different container types: polyethylene terephthalate (PET) bottles, Pyrex® dishes with a Pyrex® lid, and Pyrex® dishes covered with Saran Wrap®. This procedure was adopted to compare the wavelength effect caused by various covers on the percent photoreactivation of total coliforms.
The river water addition experiments were conducted using centrifugation (Eppendorf centrifuge 5810R, Brinkmann Instruments Inc., USA) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RPM for 45 min to separate bacteria from the effluent after applying 10 mJ cm−2 IF. The river water was filtered through a cellulose ester membrane (0.45 μm, Millipore, USA) to remove naturally occurring bacteria and was spiked with the separated bacteria to reach the same concentration as those in the original effluent. The mixture of river and effluent water with different ratios (0
000 RPM for 45 min to separate bacteria from the effluent after applying 10 mJ cm−2 IF. The river water was filtered through a cellulose ester membrane (0.45 μm, Millipore, USA) to remove naturally occurring bacteria and was spiked with the separated bacteria to reach the same concentration as those in the original effluent. The mixture of river and effluent water with different ratios (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 20
100, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 40
80, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 60
60, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 80
40, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 100
20 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) was applied for the study of photoreactivation. The irradiations followed the same procedure as described earlier. The samples were covered with two mesh filters (0.25 mm) to reduce the ERF values.
0) was applied for the study of photoreactivation. The irradiations followed the same procedure as described earlier. The samples were covered with two mesh filters (0.25 mm) to reduce the ERF values.
For all experiments, sample collection was done every half hour for the first hour and every hour for the next 3 hours. All experiments were repeated three times.
2.3. Quantitative evaluation of photoreactivation
The effects of dark reactivation and photoreactivation were evaluated by computing the percent photoreactivation, as defined by Lindenauer et al.27 as follows: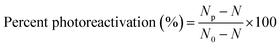 | (1) |
For all experiments, the percent dark reactivation, which varied from 0.02% (for 5 °C) to 2.5% (by using PET, Pyrex®, and Saran Wrap® filters), was subtracted from the total percent reactivation for each sample to determine the net photoreactivation.
2.4. Effective reactivation fluence (ERF) calculation method
The action spectrum reflects the relative biological or chemical photo-response per number of incident photons versus wavelength.28 The following relationship was used by Kelner et al.29 to determine relative absorption coefficients at different wavelengths: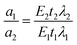 | (2) |
The same method was used by Takao et al.30 to plot the action spectra of photoreactivation for E. coli as shown in Fig. S1† by assuming the activity at 385 nm to be equal to one. These data were used to determine ERF values in this research study. First, effective spectral irradiance (ESI) values were estimated by multiplying absolute irradiance (AI) values with the average action spectrum factor (AS) values in each band. The AI values were measured by using a spectroradiometer and the AS values were estimated by the data and equation presented in Fig. S1.† The sum of the ESI values over the wavelength range of 310–480 nm gives the integrated effective irradiance (IEI). The ERF values were determined by multiplying the IEI values by time in seconds. The transmittance, attenuation factor, IEI, and ERF values of various filters are shown in Table 1. For instance, based on Table 1, for outdoor experiments without any filter the IEI is 5.2 (mW cm−2), so the ERF after 4 hours would be as follows: (5.2 × 4 × 3600)/1000 ≈ 73.7 (J cm−2).
| Filter mesh size (mm) | Number of filters | Transmittance (%) | Attenuation factor | IEI (mW cm−2) | ERF after 4 h exposure (J m−2) |
|---|---|---|---|---|---|
| — | 0 | 100.0 | 1.00 | 5.2 | 73.7 |
| 0.63 | 1 | 40.0 | 0.39 | 2.0 | 29.1 |
| 0.42 | 1 | 31.6 | 0.31 | 1.6 | 22.7 |
| 0.25 | 1 | 26.6 | 0.26 | 1.3 | 18.9 |
| 2 | 11.8 | 0.128 | 0.64 | 9.2 | |
| 3 | 2.9 | 0.030 | 0.15 | 2.2 | |
| 4 | 0.95 | 0.010 | 0.05 | 0.75 |
3. Results and discussion
3.1. Effect of fluence on inactivation and photoreactivation
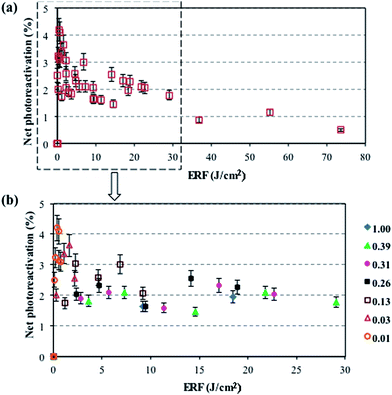
Considering the importance of the IF on photoreactivation, the effect of applied IF on photoreactivation of total coliforms was investigated. There is no significant difference between inactivation trends caused by either LP or MP UV lamps (p > 0.05) (Fig. S2†) which correlates well with previous studies.1,31 However, Guo et al.1 found a slightly lower percentage photoreactivation of total coliforms in wastewater samples after MP exposure compared to LP exposure, which validates the use of the MP lamp prior to the photoreactivation experiments. Generally, the percent photoreactivation of total coliforms decreased with increasing IF due to the increase in irreversible damage to the microorganism's cell structure.1 Based on such knowledge, and considering photoreactivation as an important issue, higher IFs are recommended.1,4,17,22,32In this study, the net percent photoreactivation of total coliforms versus ERF under actual sunlight is presented in Fig. 1. As a high solar irradiance can cause bacterial inactivation,8 various individual (and combinations of) filters with a mesh size of 0.63, 0.42, and 0.25 mm (with various attenuation factors, Table 1) were placed on the samples to reduce the inactivation effect of natural sunlight. As a result of the decreased ERF, the inactivation effect was reduced and the percent photoreactivation was enhanced by decreasing the filter mesh sizes, reaching 4% by using 4 filters with a 0.25 mm mesh size at an ERF of 1 J cm−2. As shown in Fig. 1a, bacterial reactivation occurred at ERF values lower than 30 J cm−2, while inactivation occurred at ERF values higher than 30 J cm−2 which causes negligible net photoreactivation (<1%). The same trend was observed by Bohrerova et al.8 for the photoreactivation of pure cultured E. coli ATCC 11229 after applying a 10 mJ cm−2 IF under sun lamp and sunlight exposures. In general, after applying an IF of 23 mJ cm−2, the percent photoreactivation of bacteria was less than 5% under outdoor conditions. Lindenauer et al.27 observed the same results for the percent photoreactivation of total coliforms after one hour exposure to sunlight at UV doses higher than 20 mJ cm−2. Guo et al.1 also showed that the percent photoreactivation of total coliforms is less than 5% by applying a sunlight lamp for 4 h under indoor conditions by applying UV doses more than 20 mJ cm−2. Thus, it is reasonable to consider that a low level of photoreactivation for total coliforms would exist in the GBWWTP final effluents after exposure to sunlight given a similar IF (24.2 mJ cm−2) as part of their UV disinfection process.
3.2. Effect of temperature on photoreactivation
The effect of temperature on photoreactivation under outdoor conditions and natural sunlight with an attenuation factor of 1 is shown in Fig. 2. The percent photoreactivation of total coliforms in the GBWWTP effluents increased with increasing temperature (5–25 °C) at ERF values up to a maximum of 6 J cm−2, with 0.6% at 5 °C and reached approximately 2% at 25 °C. These results correlate with the previous results which show that bacterial reactivation occurred at ERF values lower than 30 J cm−2 (Fig. 1).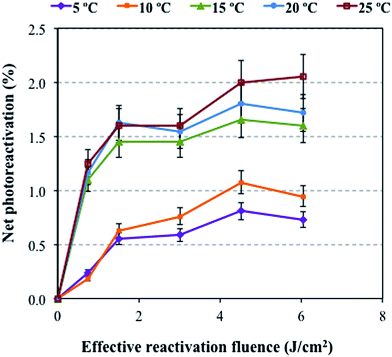 | ||
| Fig. 2 Net photoreactivation of total coliforms at various temperatures under natural sunlight with an attenuation factor of 1 after applying a 23 mJ cm−2 IF by the target WWTP. | ||
An increased value in the net photoreactivation of bacteria can be observed when temperatures increase from 10 (1%) to 15 °C (1.5%). The reason for this lies in the fact that higher temperatures make bacterial reactivation easier and extend the photoreactivation process.18 Additionally, temperatures between 15 and 25 °C are close to the optimum growth temperatures (23–37 °C) of E. coli, which increases bacterial photoreactivation.33
Another important issue is that above 15 °C there is no significant change in the percent photoreactivation of total coliforms with increasing temperature. Salcedo et al.18 also observed the same trend for the photoreactivation of total coliforms in a temperature range between 20 and 30 °C under indoor conditions by using a fluorescent lamp.
3.3. Effect of the wavelength on photoreactivation
To investigate the effect of the wavelength on the percent photoreactivation, three filters including Pyrex®, PET, and Saran Wrap® were considered by applying an IF of 10 mJ cm−2 (Fig. 3). The PET bottle blocks the wavelengths below 320 nm, but the Pyrex® and Saran Wrap® transmit almost all wavelengths of light above 300 nm (Fig. S3†). The percent photoreactivation of total coliforms increased by using the PET filter compared to Saran Wrap® and Pyrex® filters at ERF values lower than 74 J cm−2 (Fig. 3). Sinha et al.34 stated that small amounts of UV-B (280–315 nm) can induce adverse impacts on living systems. Also, Herndl et al.14 showed that bacterial activity declined by 20 to 42% after exposure to both artificial and solar UV-B radiation. In addition, molecular methods showed negligible organism damage in the samples covered with a 320 nm cut-off filter.35 Therefore, samples covered with a PET bottle, which blocks the UV-B portion of natural sunlight, show a higher percent photoreactivation compared to the other samples at ERF values lower than 74 J cm−2. However, for all filters, the percent photoreactivation decreased gradually after a 4 h light exposure (total ERF of 74 J cm−2), reaching similar levels of 4% for PET, 3% for Pyrex® and 3% for Saran Wrap®. This results from the high sunlight fluence rate that leads to bacterial inactivation after longer exposure times.8,14,20 This means that the inactivation effect of sunlight after 4 hours is such that the effect of filters becomes negligible. Interestingly, this inactivation after a longer exposure time is used in the SOlar DISinfection (SODIS) process, a process where PET bottles are used for water disinfection under natural sunlight.36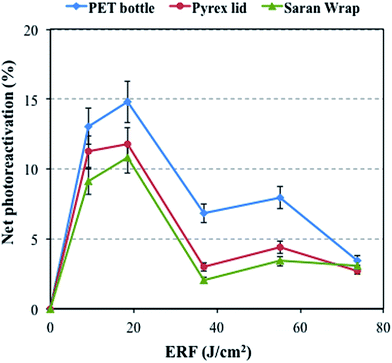 | ||
| Fig. 3 Net photoreactivation of total coliforms under natural sunlight after applying a 10 mJ cm−2 IF by using various covers. | ||
Another important issue is the slightly higher percent photoreactivation for Pyrex® compared with Saran Wrap®, which may come from the lack of the wavelength range of 200–250 nm in Pyrex® transmittance spectra.
3.4. Effect of river water addition on photoreactivation
The net percent photoreactivation of total coliforms versus the ERF in various mixtures of GBWWTP effluents and spiked filtered river water under natural sunlight after applying an IF of 10 mJ cm−2 is shown in Fig. 4. The photoreactivation of the mixtures decreased from 16% to 3% by increasing the percentage of spiked filtered river water from 0 to 100%. The reason for this finding might be attributed to the lower nutrient content in the spiked filtered river water limiting the bacterial metabolism and subsequent photoreactivation ability. The concentrations of DOC, orthophosphates, and ammonia, and alkalinity decreased on increasing the ratio of spiked filtered river water in the mixtures (Table 2). These parameters give an indication of the amount of nutrients available for bacterial growth, which impact the photoreactivation of the samples on increasing the filtered river water ratio in the mixtures. Bohrerova et al.37 also found significantly higher E. coli repair (dark and photo) in reuse water compared to drinking water and attributed it to a different water matrix and higher nutrient availability for cells in reuse water. In addition, samples including spiked filtered river water were more transparent than the GBWWTP effluent samples given the lower color and turbidity levels in the filtered river water (Table 2). This dilution effect intensifies the inactivation effect of the UV-B portion of natural sunlight and inactivation effect of high ERF (Fig. 1) on bacteria.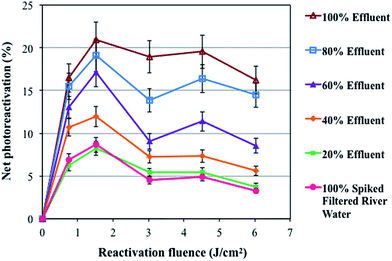 | ||
| Fig. 4 Net photoreactivation of total coliforms under natural sunlight after applying a 10 mJ cm−2 IF in mixtures of spiked filtered river water and various percentages of wastewater effluent. | ||
| Sample | DOC (mg L−1) | Orthophosphate (mg L−1) | Ammonia (mg L−1) | Color (CU) | Turbidity (NTU) | Alkalinity (mg CaCO3 per L) |
|---|---|---|---|---|---|---|
| Effluent | 17.7 ± 0.2 | 1.5 ± 0.1 | 0.11 ± 0.01 | 51.5 ± 0.1 | 10.1 ± 0.1 | 294.5 ± 0.2 |
| Spiked filtered river water | 15.5 ± 0.2 | 1.0 ± 0.1 | 0.04 ± 0.01 | 36.5 ± 0.1 | 9.6 ± 0.1 | 246.0 ± 0.2 |
| Filtered river water | 14.7 ± 0.2 | 0.8 ± 0.1 | 0.03 ± 0.01 | 31.5 ± 0.1 | 9.3 ± 0.1 | 217.0 ± 0.2 |
| 80% effluent + 20% spiked filtered river water | 17.2 ± 0.2 | 1.4 ± 0.1 | 0.10 ± 0.01 | 48.0 ± 0.1 | 10.0 ± 0.1 | 285.1 ± 0.2 |
| 60% effluent + 40% spiked filtered river water | 16.7 ± 0.2 | 1.3 ± 0.1 | 0.06 ± 0.01 | 44.5 ± 0.1 | 9.9 ± 0.1 | 273.7 ± 0.2 |
| 40% effluent + 60% spiked filtered river water | 16.3 ± 0.2 | 1.1 ± 0.1 | 0.04 ± 0.01 | 41.0 ± 0.1 | 9.8 ± 0.1 | 257.5 ± 0.2 |
| 20% effluent + 80% spiked filtered river water | 15.6 ± 0.2 | 1.0 ± 0.1 | 0.03 ± 0.01 | 37.5 ± 0.1 | 9.6 ± 0.1 | 248.0 ± 0.2 |
The results showed that adding 80% river water to the wastewater effluent decreased the percent photoreactivation of bacteria to less than 5%. Therefore, discharging the GBWWTP UV treated wastewater effluent to river water may not pose a serious risk to the receiving waters based on the impact of dilution. However, in regions where the municipal WWTP effluents have a significant volumetric input into receiving water bodies, the impact of photoreactivation may be of greater concern.
4. Conclusions
In summary, higher IFs are recommended to reduce the potential of bacterial photoreactivation in receiving waters. The effect of various filters on integrated irradiance and the attenuation factor of sunlight showed that ERF values higher than 30 J cm−2 significantly decrease the percent bacterial photoreactivation. The photoreactivation increases with increasing temperature because higher temperatures enhance the bacterial reactivation processes. In addition, the percent photoreactivation of total coliforms can increase under natural sunlight by filtering out the UV-B portion using a PET bottle filter that limits the inactivation caused by natural sunlight. It was also found that the percent bacterial photoreactivation in the treated wastewater effluent samples decreased with increasing proportions of natural river water (i.e., increasing percent dilution using natural river water) under natural sunlight.The most important finding of this research, which is applicable for the wastewater treatment industry, is that the percent photoreactivation of bacteria in a treated municipal wastewater effluent (applying about 25 mJ cm−2 IF) after a 4 h exposure to sunlight is less than 5%. Additionally, the percent photoreactivation of bacteria after mixing natural river water with the treated municipal wastewater effluent was reduced to less than 5% after applying 10 mJ cm−2 IF. Overall, this preliminary research indicates that municipal wastewater effluents treated with UV at 25 mJ cm−2 IF can be safely discharged into receiving waters.
So far, this study is the first to investigate the significant factors related to photoreactivation under natural sunlight and real outdoor conditions for an actual WWTP effluent using the ERF concept. Thus, the results of this research can be applied in the municipal wastewater treatment industry to examine the environmental effects of discharging treated municipal wastewater effluents into receiving waters.
Acknowledgements
The authors thank the financial support provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants (MGED and JRB) and an NSERC Collaborative Research and Development (CRD) Grant (MGED, JRB, and MB). Partial funding of this project was provided through the Helmholtz-Alberta Initiative (HAI). In addition, the authors thank Maria Demeter for her technical support in Dr Gamal El-Din's research laboratories in the Department of Civil and Environmental Engineering at the University of Alberta. The authors are also very grateful to EPCOR Water Services Inc. for their collaboration during wastewater sample collection.References
- M. Guo, H. Hu, J. R. Bolton and M. Gamal El-Din, Water Res., 2009, 43, 815–821 CrossRef CAS PubMed.
- S. E. Beck, R. A. Rodriguez, K. G. Linden, T. M. Hargy, T. C. Larason and H. B. Wright, Environ. Sci. Technol., 2014, 48, 591–598 CrossRef CAS PubMed.
- S. E. Beck, H. B. Wright, T. M. Hargy, T. C. Larason and K. G. Linden, Water Res., 2015, 70, 27–37 CrossRef CAS PubMed.
- E. N. Sanz, I. S. Davila, J. A. A. Balao and J. M. Quiroga Alonso, Water Res., 2007, 41, 3141–3151 CrossRef PubMed.
- C. Shang, L. M. Cheung, C. M. Ho and M. Zeng, Appl. Catal., B, 2009, 89, 536–542 CrossRef CAS.
- J. Süβ, S. Volz, U. Obst and T. Schwartz, Water Res., 2009, 43, 3705–3716 CrossRef PubMed.
- C. Hallmich and R. Gehr, Water Res., 2010, 44, 2885–2893 CrossRef CAS PubMed.
- Z. Bohrerova and K. G. Linden, Water Res., 2007, 41, 2832–2838 CrossRef CAS PubMed.
- C. Jungfer, T. Schwartz and U. Obst, Water Res., 2007, 41, 188–196 CrossRef CAS PubMed.
- C. Poepping, S. E. Beck, H. Wright and K. G. Linden, Water Res., 2014, 56, 181–189 CrossRef CAS PubMed.
- R. Buchsbaum, J. Pederson and W. E. Robinson, The decline of fisheries resources in New England: evaluating the impact of overfishing, contamination, and habitat degradation, MIT Sea Grant College Program, Massachusetts Institute of Technology, 2005 Search PubMed.
- P. Hoagland, D. Anderson, Y. Kaoru and A. White, Estuaries, 2002, 25, 819–837 CrossRef.
- H. Liltved and B. Landfald, Water Res., 2000, 34, 481–486 CrossRef CAS.
- G. J. Herndl, A. Brugger, S. Hager, E. Kaiser, I. Obernosterer, B. Reitner and D. Slezak, Plant Ecol., 1997, 128, 42–51 Search PubMed.
- L. W. Sinton, R. J. Davies-Colley and R. B. Bell, Appl. Environ. Microbiol., 1994, 60, 2040–2048 CAS.
- Y. Y. Chan and E. G. Killick, Water Res., 1995, 29, 1373–1377 CrossRef CAS.
- A. Locas, J. Demers and P. Payment, Can. J. Microbiol., 2008, 54, 971–975 CrossRef CAS PubMed.
- I. Salcedo, J. A. Andrade, J. M. Quiroga and E. Nebot, Appl. Environ. Microbiol., 2007, 73, 1594–1600 CrossRef CAS PubMed.
- J. Hu and P. H. Quek, Appl. Environ. Microbiol., 2008, 74, 327–328 CrossRef CAS PubMed.
- C. G. Yoon, K. W. Jung, J. H. Jang and H. C. Kim, Paddy Water Environ., 2007, 5, 57–62 CrossRef.
- G. D. Harris, D. Adams, D. L. Sorensen and M. S. Curtis, Water Res., 1987, 21, 687–692 CrossRef CAS.
- W. A. M. Hijnen, E. F. Beerendonk and G. J. Medema, Water Res., 2006, 40, 3–22 CrossRef CAS PubMed.
- O. Hoyer, Water Supply, 1998, 16, 419–424 Search PubMed.
- K. Tosa and T. Hirata, Water Res., 1999, 33, 361–366 CrossRef CAS.
- APHA, AWWA and WEF, Standard Methods for the Examination of Water and Wastewater, America Public Health Association, Washington, D.C., 21st edn, 2005 Search PubMed.
- J. R. Bolton and K. G. Linden, J. Environ. Eng., 2003, 129, 209–215 CrossRef CAS.
- K. G. Lindenauer and J. L. Darby, Water Res., 1994, 28, 805–817 CrossRef CAS.
- J. R. Bolton, Ultraviolet Applications Handbook, ICC Lifelong Learn Inc., Edmonton, Canada, 3rd edn, 2010 Search PubMed.
- A. Kelner, J. Gen. Physiol., 1951, 34, 835–852 CrossRef CAS PubMed.
- M. Takao, A. Oikawa, A. P. Eker and A. Yasui, Photochem. Photobiol., 1989, 50, 633–637 CrossRef CAS PubMed.
- A. C. Eischeid and K. G. Linden, J. Appl. Microbiol., 2007, 103, 1650–1656 CrossRef CAS PubMed.
- J. L. Zimmer and R. M. Slawson, Appl. Environ. Microbiol., 2002, 68, 3293–3299 CrossRef CAS PubMed.
- P. Quek and J. Hu, J. Appl. Microbiol., 2008, 105, 124–133 CrossRef CAS PubMed.
- R. P. Sinha and D.-P. Häder, Photochem. Photobiol. Sci., 2002, 1, 225–236 CAS.
- D.-P. Häder and R. P. Sinha, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2005, 571, 221–233 CrossRef PubMed.
- J. A. Byrne, P. A. Fernandez-Ibañez, P. S. Dunlop, D. Alrousan and J. W. Hamilton, Int. J. Photoenergy, 2011, 2011, 1–12 CrossRef.
- Z. Bohrerova, J. Rosenblum and K. G. Linden, J. Environ. Eng., 2014, 141, 04014094 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed methodology for the effect of inactivation fluence, sample calculation of effective reactivation fluence, and transmittance spectra of various filters. See DOI: 10.1039/c6em00501b |
| This journal is © The Royal Society of Chemistry 2017 |